Nonspecific Orbital Inflammation (Idiopathic Orbital Inflammation, Orbital Inflammatory Syndrome, Orbital Pseudotumor)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Nonspecific orbital inflammation (NSOI), also known as orbital inflammatory pseudotumor, idiopathic orbital inflammation (IOI) and orbital inflammatory syndrome is the most common cause of painful orbitopathy in adults.[1] NSOI can be localized or diffuse. When localized, inflammation can affect the extraocular muscles (orbital myositis), lacrimal gland (dacryoadenitis), sclera (scleritis), uvea (uveitis), and the superior orbital fissure and cavernous sinus (Tolosa-Hunt syndrome). Others include periscleritis, perineuritis, and an isolated orbital mass. When diffuse, NSOI may diffusely involve the orbital fatty tissues. NSOI is the third most common orbital disease after thyroid eye disease and orbital lymphoma.[1] NSOI can both radiologically and clinically mimic a malignant process. Therefore, it is a diagnosis of exclusion, only after all other causes of inflammation have been eliminated. Treatment options are varied and can include surgery, steroids, chemotherapeutic agents and irradiation.[2]
Disease Entity
International Classification of Disease (ICD)
ICD-9-CM 376.11 Orbital granuloma
ICD-10-CM H05.11 Granuloma of orbit
History
Nonspecific orbital inflammation was first described in 1905 by Birch-Hirschfeld.[3] It was named as inflammatory pseudotumor in 1954 by Umiker et al.[4] because of its propensity to mimic a malignant process. Presently, Nonspecific orbital inflammation and orbital inflammatory pseudotumor can be used interchangeably.
Definition
NSOI is a benign noninfectious, inflammatory process of the orbit ; characterized by a polymorphous lymphoid infiltrate with varying degrees of fibrosis, without a known local or systemic cause.[5] h
Incidence
The true incidence of NSOI is difficult to assess given the wide range of manifestation and lack of universally accepted definition. NSOI has been shown to account for up to 8-10 % of orbital disorders.[6]
Frequency of NSOI subtypes
Swamy et al.[7] reviewed 24 patients with biopsy proven NSOI and found that the lacrimal gland was affected 54.2% of the time (13/24), extraocular muscles 50.0% (12/24), orbital fat 75.0% (18/24), sclera 4.2% (1/24), optic nerve 20.8% (5/24) and other 8.3% (2/27).
Histopathology
The histopathologic spectrum of NSOI is typically non-diagnostic and diverse. The pathogenesis is controversial due to the wide range of presentation ranging from the typical diffuse polymorphous infiltrate to lymphoid, granulomatous, sclerosing, eosinophilic or vasculitic inflammation.[8] Reese presented 5 subdivisions[9] and Farrow proposed 2 classes.[10] However, to date, no one classification scheme has been universally accepted.
Pathophysiology
The etiology and pathogenesis of NSOI is currently unknown. Both infectious and immune-mediate etiologies have been implicated. Other suggested theory is molecular mimicry when a foreign antigen structural has similarities with self-antigens may happen after an acute infection. NSOI has been observed in association with a variety or rheumatologic conditions including Crohn's disease, systemic lupus erythematous, rheumatoid arthritis, myasthenia gravis, and ankylosing spondylitis.[8][11][12][13][14] Purcell and Taulbee[15] reported a case of new onset orbital myositis within two weeks after confirmed streptococcal pharyngitis. Mombaerts et al.[16] found in their series that 10% of their patients with NSOI also had a concurrent autoimmune disease. In another study by Sobrin et al.[17], 21 of 27 patients that were treated with infliximab for ocular inflammation were found to have coincidental rheumatologic disease. Atabay et al.[18] reported that circulating antibodies against eye muscle antigens are present in patients with orbital myositis. They found autoantibodies active against eye muscle membrane proteins of 55 and 64 kilodaltons which were seen in 63% of the orbital myositis patients compared with 16-20% in healthy patients. It has been proposed that this autoimmunity may be the ocular mechanism for some forms of orbital myositis. However, antibodies to this protein have also been seen in thyroid orbitopathy.[18] Additionally, the typical unilateral presentation of NSOI argues against this type of autoimmunity as being the primary mechanism of NSOI.
Mottow-Lippe et al.[19] suggest that trauma may cause increased vascular permeability resulting in release of antigenic substances which in turn incite an inflammatory cascade. They propose that the variable nature and multi-focality of NSOI can be explained by the network of connective tissue and capillaries delivering antigenic agents to a variety of orbital structures. Wladis et al.[20] performed quantitative cytokine assays for 9 different molecules and noted that six cytokines were significantly elevated in NSOI (interleukin-2, -8, -10, -12, gamma interferon, and tumor necrosis factor alpha). An animal model has been proposed however more complete models are needed to better understand NSOI pathophysiology and optimum treatment protocol.[21] Interference of CD20, CD25 and Toll-like receptors may provide the basis for future therapies.[22] An immune-mediated pathophysiology is strongly suggested by increased cytokines and favorable, rapid responses of inflammation to systemic corticosteroids and other immunosuppressive agents. These therapies will be discussed further in the management section.
Diagnosis
Clinical Presentation
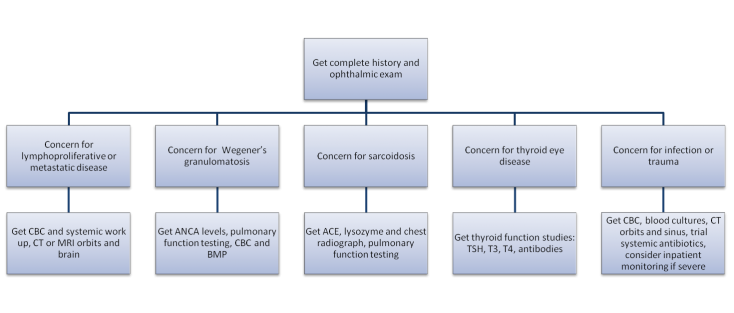
All patients with suspected NSOI require a full ophthalmic assessment/workup. NSOI is typically characterized by the abrupt onset of pain, proptosis and other orbital inflammatory signs such as swelling and erythema. Unilateral presentation is more typical but bilateral presentations are not uncommon.[23] Pediatric NSOI differs from the adult presentation and is more commonly characterized by bilateral manifestation, uveitis, disc edema and eosinophilia.[24] Pain is the most common symptom in adult NSOI and occurs 58-69% of the time followed by diplopia (31-38%).[24][25] Periorbital edema/swelling is the most common sign and occurs 75-79.2% of the time (figure) followed by proptosis (32-62.5%), EOM restriction (54.2%) red eye (48%), chemosis (29%), decreased vision (20.8%), and ptosis (16.7%).34-35 Therefore, physical examination of patients with suspected NSOI involves lid assessment (retraction/lid lag/lagophthalmos), orbital assessment (proptosis), extraocular muscles (restriction), globe (injection/chemosis), and optic nerve function (visual acuity/color plates/relative afferent pupillary defect). Because of the association between rheumatologic disease and NSOI the typical laboratory work-up for suspected NSOI should include a complete blood count, basic metabolic panel, thyroid function studies, erythrocyte sedimentation rate, antinuclear antibodies, antineutrophil cytoplasmic antibodies, angiotensin-converting enzyme level, rapid plasma reagin test, and rheumatoid factor.[8] Additionally, infectious etiologies including syphilis and tuberculosis should be ruled out.
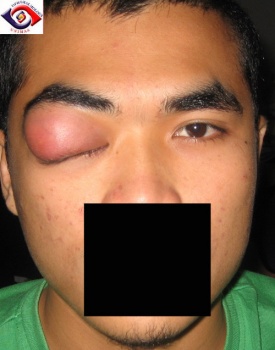
Figure 1: Dense upper eyelid edema and swelling in a patient with lacrimal gland NSOI, Courtesy of Professor M Chua
Imaging
Evaluation of NSOI will frequently involve high-resolution computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI). CT allows for orbital bony and sinus evaluation,[2] whereas MRI superiorly demonstrates soft tissue changes in the region of the cavernous sinus/superior orbital fissure.[26] Kapur et al.[27] reported different intensity patterns on diffusion-weighted imaging (DWI) between NSOI, orbital cellulitis and orbital lymphoma, and thus DWI may aid in differentiation.[27] Arterial spin labeling (ASL) may also be useful in differentiating between NSOI and lymphoproliferative malignancy.[28] Radiologic findings allow subtypes of NSOI to be more precisely classified and are as follows:[1][25][29][30][31][32]
Lacrimal gland
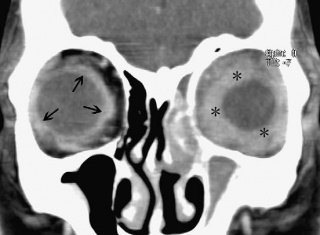
The lacrimal gland will appear diffusely enlarged with overall preservation of its shape. There may be blurring at the gland margin with marked expansion along the lateral orbital wall and lateral rectus muscle (Figure 2).
Figure 2: Diffusely enlarged right lacrimal gland with blurring of gland margin. Courtesy of Professor M Chua
Extraocular muscles
Enlargement of the extraocular muscles will be seen (single or multiple). Unilateral single muscle inflammation with tendon involvement is most common. The most frequently involved muscle is the medial rectus followed by the superior rectus, lateral rectus and inferior rectus. The tendon often also enlarges with the muscle belly, unlike in thyroid orbitopathy in which the tendon is classically "spared" (Figure 3). There may be infiltrates throughout the orbital fat bordering the muscle, blurring the margin of the muscle.
Figure 3: CT image of bilateral medial and lateral rectus tubular-like enlargement with tendon involvement. Courtesy of Z.X. Ding
Optic nerve
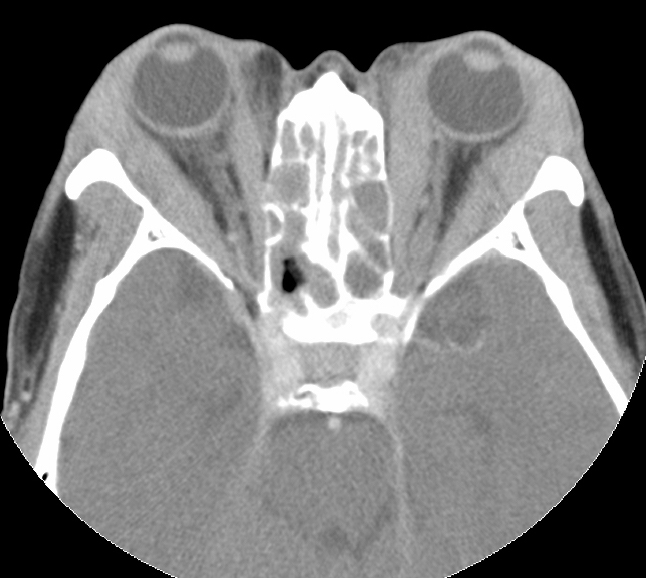
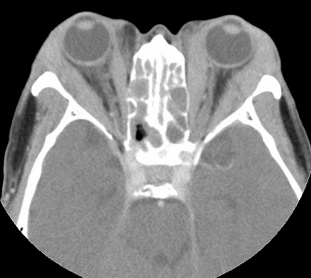
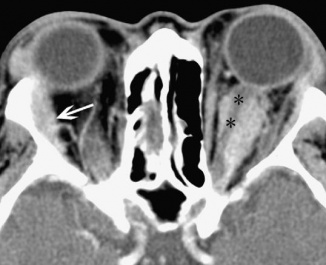
Inflammatory tissues surrounding an unenhanced optic nerve may demonstrate the classical, "tramline" sign (Figure 4). There may be streaky densities in the contiguous orbital fat.
Figure 4: CT image of optic nerve involvement with sheath enhancement ("tramline" sign) (asterisks), white arrow showing right lacrimal gland enlargement. Courtesy of Z.X. Ding
Sclera, episclera, Tenon's capsule, and uvea:
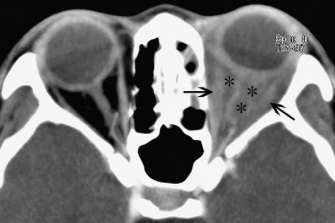
Imaging will demonstrate non-specific thickening of structures. Blurring of the sclera margin may be seen (Figure 5).
Figure 5: CT image showing thickening and blurring of left eye uveoscleral (asterisks). Courtesy of Z.X. Ding
Orbital fat
Diffuse infiltration and inflammation will be seen in the orbital fat and may envelop the globe and optic nerve sheath complex (Figure 6).
Figure 6: CT image showing enhancement of orbital fat (asterisks). Courtesy of Z.X. Ding
Orbital apex, cavernous sinus and intracranial involvement:
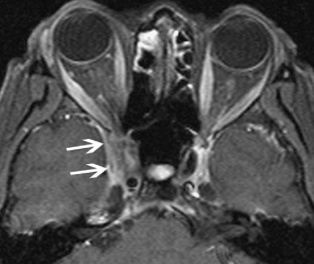
There may be compression or displacement of the optic nerve. The cavernous sinus (Figure 7) and middle cranial fossa are the two most common locations for intracranial extension of NSOI. Intracranial involvement can feature abnormal soft tissue in the superior orbital fissure, expansion of the ipsilateral cavernous sinus and thickening of the meninges contiguous with the orbital inflammation.
Figure 7: MRI, fat-saturated, T1-weighted image with white arrows showing extension into the cavernous sinus. Courtesy of Z.X. Ding
Biopsy
The necessity for biopsy is debated. There may be not be a distinct mass to biopsy or the lesion may be unapproachable and response to therapy can be confirmatory.[33] Biopsy may be considered if there are progressive neurologic deficits, lack of steroid responsiveness and persistent imaging abnormalities.
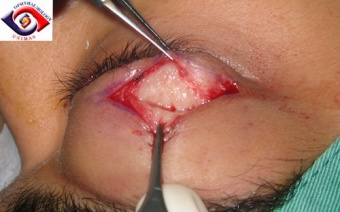
Figure 8: Showing whitish infiltration of the lacrimal gland on direct upper eyelid incision, Courtesy of Professor M Chua
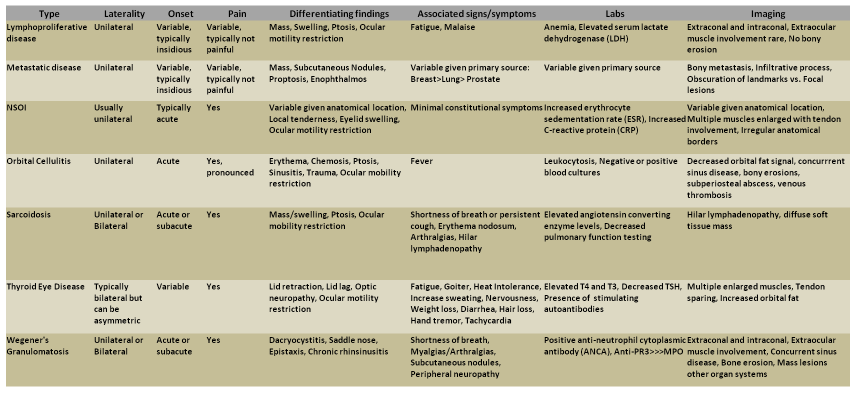
Differential Diagnosis
There are many processes that can mimic NSOI. The most common orbital processes that present with similar clinical pictures as NSOI are thyroid eye disease and orbital cellulitis.9 Thyroid eye disease is the most common cause of orbital inflammation in adults and has been found to account for nearly 60% of cases of orbital inflammation in the 21-60 year old age group.[34] Orbital cellulitis risk factors include history of sinusitis, dental work/disease, or trauma.[35][36] Table 1 outlines common differential diagnosis for NSOI.
Table 1: Differential Diagnosis of Orbital Inflammation[8][25][33][36][37][38][39][40][41][42][43]
Management
Observation
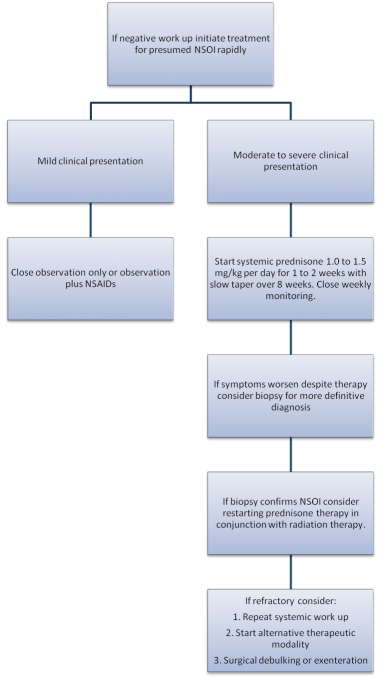
Observation for NSOI for mild cases of inflammation may be acceptable. Swamy et al.[7] reviewed the treatment of 24 NSOI patients with a minimum 6 month follow-up and found that 20.8% (5/24) that were treated with observation alone had maintained remission. If there is no clinical resolution or worsening of symptoms then additional therapy is indicated.
Non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs, such as ibuprofen, have been used in mild cases of NSOI. There has been no formal study evaluating the use of NSAIDS in NSOI. Mannor et al.[44] reported that NSAIDs could be used up to 3 weeks as long as clinical resolution was being observed, with steroids reserved for refractory cases. The side effects to NSAIDs are dose-dependent with an estimated 10-20% of NSAID patients experiencing dyspepsia which can be reduced through suppressing acid production, via a proton pump inhibitor, e.g. omeprazole or esomeprazole.[45]
Corticosteroids
Systemic corticosteroids are generally considered the gold standard treatment for NSOI with its both anti-inflammatory effect ( by inhibition of phospholipase A2 and cyclooxygenase pathways) and immunosuppressive effect ( by inhibition of IL, IFN synthesis,cytotoxic effect on T lymphocytes, and inhibition of major histocompatibility antigen expression) .[8][33] Typically, response to steroids is rapid with a dramatic improvement in all symptoms and findings. In their review of 65 NSOI patients, Yuen and Ruben[23] found that 69% were treated with steroids alone, 12% with steroids and radiation therapy and 9% with steroids and NSAIDs. Yuen and Ruben[23] also noted that 24 patients had treatment failures with steroid dependence and steroid intolerance occurring 33% and 13% of the time, respectively.[46][47] Treatment doses can differ in range but are generally 1.0-1.5 mg/kg or 50-100 mg/day for 1-2 weeks followed by slow taper for 5-8 weeks. It is important to counsel patients about need for extended steroid taper, as short tapers are highly associated with disease relapse, and to encourage prophylaxis for steroid-related side effects including gastritis/GERD, insomnia, weight gain, and mood changes.
Radiation therapy
External beam radiotherapy may be used in the treatment of NSOI as an alternative or adjuvant. It is generally used when NSOI is found to be resistant to or intolerant to corticosteroid therapy.[46] The results of radiotherapy have been reported to have success rates ranging between 50-75%.[48][49] Other authors have published greater long-term control to ranging from 66%-100% success after a total dosage of 2000 cGy.[48][50] In their review of 24 NSOI patients treated with radiation therapy, Lanciano et al.[48] found that 87% of patients had soft tissue swelling improvement, 82% with improvement in proptosis, 78% with improved ocular motility restriction and 75% had decreased pain. Kennerdell et al.[51] showed benefit at doses of 2500 to 3000 cGy over ten days, Sergott et al.[52] at doses of 1000 to 2000 cGy over 10-15 days and Orcutt et al.[49] at 2500 cGy over 15 days. Surveillance for radiation-induced ophthalmic side effects, including dry eye, periocular dermatitis, keratitis, cataract, optic neuropathy and retinopathy should be performed.
Calcineurin inhibitors:
Cyclosporine-A (CsA)
CsA is an im
munosupressent that acts on T-lymphocytes. It inhibits synthesis T-cell growth cytokines, IL-2 and IFN-γ.[53] A few studies have shown that cyclosporine can be efficacious in diabetic NSOI patients who cannot tolerate steroids.[2] Diaz-Llopis and Menezo[54] recommend treating NSOI with 5mg/kg/day then tapering to 2mg/kg/day over ten months. Zacharopoulos et al.[55] treated a patient using 4mg/kg/day for 6 weeks and this patient remained symptom free for 5 years without any medication.
Tacrolimus
Tacrolimus (Fk506) is very similar to cyclosporine but is approximately 10 times more potent.[56] Tacrolimus has been shown to be useful for ocular immunosupression,[57][58] but literature regarding treatment of NSOI has been very limited.
Antiproliferative drugs (Cytotoxic):
Azathioprine
Azathioprine is a mercaptopurine analong which inhibits purine metabolism enzymes. There are only case reports regarding azathioprine treatment for NSOI. Garrity et al.[47] noted that azathioprine was ineffective in a patient with vasculitic NSOI, however, Rootman et al.[46] found azathioprine useful in one patient in conjunction with systemic corticosteroids.
Cyclophosphamide
Cyclophosphamide is a B-cell cytotoxic alkylating agent.[59] There are only limited case reports of cyclophosphamide used in NSOI. Paris et al.[60] reported effectiveness with pulsed cyclophosphamide combined with prednisone. Other cases reports have demonstrated durable anti-inflammatory effect for up to 7 years.[47][60][61] In cases of sclerosing NSOI, Winn and Rootman[62] noted one patient with improvement with cyclophosphamide but with recurrence 6 years later, another patient with good response to cyclophosphamide, one that developed recurrence but achieved control with cyclophosphamide, and one that appeared stable with cyclophosphamide plus colchicine. Adverse drug reactions include nausea and vomiting, bone marrow suppression, gastrointestinal distress, diarrhea, alopecia and lethargy.[60][61] Hemorrhagic cystitis can also occur but is prevented by fluid intake and mesna.[63] A long-term complication can be the development of transitional cell carcinoma of the bladder.[63]
Methotrexate
Methotrexate is an inhibitor of dihydrofolate reductase, an enzyme needed in folic acid synthesis. This results in suppression of both T-cell and B-cell functions. Methotrexate is also known to enhance the release of adenosine, which has potent anti-inflammatory effects.[64] Methotrexate has a long history of success in the treatment of rheumatoid arthritis.[33] For ocular immunosupression Hemady et al[53] recommend 10 to 25 mg divided over 36 to 48 hours every 1 to 4 weeks. Smith and Rosenbaum[65] reported treating seven NSOI patients with methotrexate ranging from 15 to 25 mg/week for periods of 4 weeks to 34 months. Of those seven patients 4 demonstrated clinical benefit, in one patient methotrexate was stopped due to side effects, in one there was no response, and 2 patients did not complete the 4 months trial for undisclosed reasons. Shah et al.[66] evaluated methotrexate use in NSOI at low doses of 12.5 mg/wk and reported 16 out of 22 patients that had a reduction of inflammatory activity. Fourteen of the 16 patients were able to taper or discontinue corticosteroid therapy and 5 patients had complete remission. Six patients did not response to methotrexate. Prominent side effects of methotrexate include gastrointestinal disturbances, arthralgias, liver abnormalities, alopecia, fatigue and headache.[8][64][59][56][65] Dietary supplements of folate, restriction of alcohol intake and parenteral administration of methotrexate can prevent these side effects.
Cytokine/protein specific biologic agents:
Adalimumab
Adalimumab is a recombinant IgG1 monoclonal antibody containing 100% human peptide sequences targeting tumor necrosis factor alpha (TNF-α). TNF-α is a cytokinic key factor in the inflammatory cascade. Adalimumab has been proven to be effective in adult patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis.[67][68][69][70] The results of etanercept for eye related diseases have been mixed. In a randomized, double blind trial of 18 patient with ocular sarcoidosis there was found to be no therapeutic benefit over placebo.[71] A similar results was reported in the treatment of JIA uveitis.[72] The Wegener's Granulomatosis Etanercept Trial (WEGET) was a randomized, placebo-controlled trial where etanercept was evaluated for maintenance of remission in 180 patients. The study found that etanercept was ineffective, but also those treated with etanercept were found to have a higher risk of developing solid tumors in comparison with those treated with cyclophosphamide.[73]
Infliximab
Infliximab is a chimeric monoclonal antibody against TNF-α. As infliximab is one of the first specific agents to be directed against TNF-α, there has been more evidence regarding its use in variety of ocular disease. It has been so successful in Behçet's disease that it is becoming the treatment of choice for this disorder.[74][75] There has been increasing body of evidence that infliximab is a useful therapeutic option in NSOI. Garrity et al.[76] reported the treatment of 7 patients with chronic and refractory orbital myositis. Patients received a dosing schedule of 3 to 5 mg/kg (up to 10 mg/kg) given at weeks 0,2,and 6 with treatments every 4 to 8 weeks afterwards. It was noted that all 7 patients had a favorable response to treatment with no untoward effects after a mean follow-up of 15.7 months (range, 4 to 31 months). Miguel et al.[77] has reported two cases of steroid dependent NSOI who developed adverse effects from conventional steroid-sparing agents, in both cases symptoms had disappeared with infliximab with follow-up of at least 20 months. Sahlin et al.[78] described successful treatment of 1 patient with sclerosing NSOI with combination infliximab and methotrexate therapy. Wilson et al.[79] has reported success in the treatment in a pediatric patient with refractory bilateral NSOI and has remained symptom free and off corticosteroids 2 years since initial diagnosis Side effects include rash, headache, respiratory congestion, hypotension, development of autoantibodies and possible risk of lymphoma.[80][81][82]
Rituximab
Rituximab is a chimeric mouse-human monoclonal antibody against the protein CD20, which is primarily found on B-cells as a cell-surface protein. Although ritixumab is a monoclonal antibody it tends to act more as a cytotoxic agent than other biologic agents.[59]On et al.[83] first reported the use of rituximab in the successful management of one patient with refractory NSOI in combination with CyberKnife radiosurgery with rituximab dosing at 375mg/m2 IV weekly for 4 weeks. Schafranksi[84] reported success with rituximab in one patient with NSOI refractory to azathioprine therapy, at dosing of two 1000-mg infusions on days 0 and 15. Lastly, Ibrahim et al.[85] has reported successful treatment one patient with rheumatoid arthritis who developed NSOI refractory to adalimumab with ritixumab dosing of 1000-mg infusions administered 2 weeks apart. The side effects of rituximab include infusion site reactions, rash, rigors, fever, headache, infection, and bronchospasm.[59][86]
Tocilizumab
Tocilizumab is an anti-interleukin-6 receptor antibody that has been shown to be effective in the treatment of systemic-onset juvenile idiopathic arthritis and rheumatoid arthritis.[87][88] A review of 392 patients with noninfectious anterior scleritis showed 1 patient with successful treatment of scleritis with Tocilizumab.[89] Tappeiner et al.[90] reported 3 patients with JIA-associated uveitis that were treated with tocilizumab. Two out of the three patients achieved inactivity of uveitis while 1 patient required increased in the osage of topical steroids. In all three patients arthritis improved. To date toclizumab has not been reported in treating NSOI. The most common side effects of tocilizumab include upper respiratory tract infections, nasopharyngitis, headache, hypertension, and transient elevation of serum liver enzyme levels.[91] More serious side effects include neutropenia, serious infection, and thrombocytopenia.[92]
Intravenous Immunoglobulin and Plasmapheresis
Both intravenous immunoglobulin (IVIG) and plasmapheresis act via removal of autoantibodies by neutralization and filtration, respectively.[56] However, the exact mechanism of action of IVIG in immune-mediated diseases remains unknown.[93] It has been suggested that IVIG may activate the inhibitory Fc receptor pathway.[94] In one study, Rosenbaum et al.[95] treated 10 patients with refractory bilateral uveitis with IVIG and observed sustained and substaintial benefit in 5 out of the 10 patients for over 11 months. Shambal et al.[96] treated 1 patient with refractory orbital myositis with .3 g/kg/weight for 3 days and considerable improvement was noted. Symon et al.[97] reported the successful treatment of resistant NSOI with a total dose of 2g/kg divided over 4 days as an 8-hour infusion with resolution of pain and proptosis. IVIG has also been used successfully in the thyroid eye disease.[98] There have been no case reports of the use of plasmapheresis in the treatment of NSOI. IVIG is associated with thromboembolism, aseptic meningitis and the risk of transmission of blood-borne infection.[93] Despite the promise, IVIG is a limited resource and, therefore, is an extremely costly therapy. As a result its use should probably be limited to those who have failed virtually all other available treatments.[93]
Surgical Therapy
While biopsy may be helpful in establishing a diagnosis, surgical resection is not typically a primary treatment for NSOI due to its often diffuse nature and response to anti-inflammatory therapy. In an eye with a confirmed diagnosis of NSOI that becomes blind and painful or is completely refractory to all treatments exenteration may be considered.[23]
Decision Tree
Step 1: If negative proceed to step 2
Step 2
Outcomes
Outcomes regarding NSOI differ in the literature given the variability in both disease presentation and treatment protocols. Retrospective data from academic centers may reflect an overall higher rate of corticosteroid failures than observed in the community as these centers will generally see more severe or recalcitrant disease.
In 2002, Yuen and Ruben[23] reviewed 65 NSOI patients who were treated at the Massachusetts Eye and Ear Infirmary from January 1991 to April 2001. Treatment modalities used included steroids, steroids and radiation, steroids and NSAIDs, radiation and NSAIDs, NSAIDs alone, surgical debulking and observation only. Five years after Yuen and Rubin, Swamy et al.[7] published treatment outcomes of 24 patients with biopsy proven NSOI. Therapeutic modalities included observation alone, antibiotics, oral corticosteroids, intravenous corticosteroids, adjunctive radiation therapy and systemic immunosuppressive drugs (methotrexate, azathioprine, mycophenolate, and ciclosporine). Of the 24 patients, 16 (67%) had complete resolution of symptoms, 4 (17%) had partial resolution and 4 (17%) had no improvement in their symptoms. In 2012 Pemberton and Fay[99] reviewed all published cases of sclerosing NSOI. Seventeen articles with 56 biopsy-proven sclerosing NSOI with documented outcomes were reviewed. There were 15 different treatment regimens including steroids, radiation therapy and immunomodulatory drugs. Regardless of treatment modality the overall response was good in 19 (34%) patients, partial in 24 (43%), and poor in 13 (23%).
Discussion
NSOI is diagnosis of exclusion and is highly variable. Given the variable nature of the disease and the emergence of immunosuppresive drugs many therapeutic regimens exist. Retrospective studies have demonstrated that patients on average have symptomatic improvement. It is generally agreed upon that steroids are the initial treatment of choice for moderate to severe NSOI.
Additional Resources
1. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002590/
2. http://www.nlm.nih.gov/medlineplus/ency/article/001623.htm
3. http://en.wikipedia.org/wiki/Idiopathic_orbital_inflammatory_disease
References
- ↑ 1.0 1.1 1.2 Weber AL, Romo LV, Sabates NR. Pseudotumor of the orbit: clinical, pathologic, and radiologic evaluation. Radiol Clin North Am 1999;37:151-168
- ↑ 2.0 2.1 2.2 Narla LD, Newman B, Spottswood SS, Narla S, Kolli R: Inflammatory Pseudotumor. Radiographics, online publication 2003;23:719-720
- ↑ Birch-Hirschfeld A. Zur Diagnostic and Pathologic der Orbital Tumoren. Bericht uber die Zusammenkunft der Deutschen Ophthalmologischen Gesellschaft 1905;32:127e35.
- ↑ Umiker WO, Iverson LC. Post inflammatory tumor of the lung: report of four cases simulating xanthoma, firboma, or plasma cell granuloma. J. Thorac Surg 1954;28:55-62
- ↑ Orbits, Eyelids, and Lacrimal System, AAO, BCSC Section 7, 2011-2012 pg. 59
- ↑ Yuen SJ, Rubin P. Idiopathic orbital inflammation: ocular mechanisms and clinicopathology. Ophthalmol Clin N Am 2002;15:121-126
- ↑ 7.0 7.1 7.2 Swamy BN, McCluskey P, Nemet A, Crouch R, Martin P, Benger R, Ghabriel R, Wakefield D. Idiopathic orbital inflammatory syndrome: Clinical features and treatment outcomes. Br J Ophthalmol 2007;91:1667-1670
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Espinoza GM. Orbital Inflammatory Pseudotumors: Etiology, Differential, Diagnosis, and Management. Curr Rheumatol Rep 2010;12:443-447
- ↑ Reese AB. Tumors of the eye. New York: Harper; Row; 1951. p. 518
- ↑ Henderson JW, Farrow GM, editors. Orbital Tumors. Philadelphia: W.J. Saunders Co.; 1973. p.555
- ↑ Smith JW. Orbital pseudotumor and Crohn’s disease. Am J Gastroenterol 1992;87(3):405-406
- ↑ Serop S, Vianna RN, Claeys M, DE Laey JJ. Orbital myositis secondary to systemic lupus erythematosus. Acta Ophthalmol 1994;72:520-523
- ↑ Van de Mosselaer G, Deuren HV, Dewolf-Peeters C, et al. Pseudotumor orbitae and myasthenia gravis. Arch Ophthalmol 1980;98:1621-1622
- ↑ Woo TL, Francis IC, Wilcsek GA, Coroneo MT, McNab AA, Sullivan TJ. Australian orbital and adnexal Wegner’s granulomatosis. Ophthalmology. 2001;108: 1535-1543
- ↑ Purcell JJ, Taulbee WA. Orbital myositis after upper respiratory tract infection. Arch Ophthalmol 1981;99:437-438
- ↑ Mombaerts I, Koornneef L. Current status in the treatment of orbital myositis. Ophthalmology 1997;104(3):402-408
- ↑ Sobrin L, Kim E, Christen W et al. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125:895-900
- ↑ 18.0 18.1 Atabay C, Tyutyunikov A, Scalise D, et al. Serum antibodies reactive with eye muscle membrane antigens are detected in patients with nonspecific orbital inflammation. Ophthalmology. 1995;102:145-153
- ↑ Mottow-Lippa L, Jakobiec FA, Smith M. Idiopathic inflammatory orbital pseudotumor in childhood II results of diagnostic tests and biopsies. Ophthalmology. 1981;88(6):565-574
- ↑ Wladis EJ, Iglesias BV, Gosselin EJ. Characterization of the Molecular Biologic Milieu of Idiopathic Orbital Inflammation. 2011;27:251-254
- ↑ Fries PD, Fohrman D, Char DH. Phorbol ester-induced orbital myositis. Arch Ophthalmo.l 1987;105:1273-1276
- ↑ Wladis EJ, Iglesias BV, Adam AP, Nazeer T, Gosselin EJ. Toll-Like Receptors in Idiopathic Orbital Inflammation. Ophthal Plast Reconstr Surg. 2012;28:273-276
- ↑ 23.0 23.1 23.2 23.3 23.4 Ahn Yuen SJ, Rubin PAD. Idiopathic Orbital Inflammation Distribution, Clinical Features, and Treatment Outcome. Arch Ophthalmol. 2003;121:491-499
- ↑ 24.0 24.1 Berger JW, Rubin PAD, Jakobiec FA. Pediatric orbital pseudotumor: case report and review of the literature. Int Ophthalmol Clin 1996;36:161-177
- ↑ 25.0 25.1 25.2 Ding ZX, Lip G, Chong V. Idiopathic orbital pseudotumor. Clinical Radiology 2011;66:886-892
- ↑ Kline LB, Hoyt WF. The Tolosa-Hunt syndrome. J Neurol Neurosurg Psychiatry 2001;71:577-582
- ↑ 27.0 27.1 Kapur R, Sepahdari AR, Mafee MF, et al. MR imaging of orbital inflammatory syndrome, orbital cellulitis, and orbital lymphoid lesions: the role of diffusion-weighted imaging. AJNR Am J Neuroradiol 2009;30:64-70
- ↑ Eissa L, Abdel Razek AAK, Helmy E. Arterial spin labeling and diffusion-weighted MR imaging: Utility in differentiating idiopathic orbital inflammatory pseudotumor from orbital lymphoma. Clin Imaging. 2021 Mar;71:63-68. doi: 10.1016/j.clinimag.2020.10.057. Epub 2020 Nov 6. PMID: 33171369.
- ↑ Flanders Ae, Mafee MF, Rao VM, et al. The characteristics of orbital pseudotumor and other orbital inflammatory processes. J Comput Assist Tomogr 1989;13:40-47
- ↑ McKinney AM, Short J, Lucato L, et al. Inflammatory myofibroblastic tumor of the orbit with associated enhancement of the meninges and multiple cranial nerves. Am J Neuroradiol 2006;27:2217-2220
- ↑ Lee EJ, Jung Sl, Kim BS, et al. MR imaging of orbital inflammatory pseudotumors with extraorbital extension. Korean J Radiol 2005;6:82-88
- ↑ Clifton AG, Borgstein RL, Moseley IF, et al. Intracranial extension of orbital pseudotumor. Clin Radiol 1992;45:23-26
- ↑ 33.0 33.1 33.2 33.3 Jacobs D, Galetta S. Diagnosis and management of orbital pseudotumor. Curr Opin Ophthalmol 2002;13:347-351
- ↑ Dutton JJ. Orbit and lacrimal gland. In: Yanoff M, Duker JS, editors. Ophthalmology. Mosby, Inc., St. Louis, 2004, p.729-743
- ↑ Israele V, Nelson JD. Periorbital and orbital cellulitis. Ped Infect Dis J 1987;6:404-410
- ↑ 36.0 36.1 Mawn LA, Jordan DR, Donahue SP. Preseptal and Orbital Cellulitis. Ophthalmol Clin N Am 2000;13:633-41
- ↑ Lutt JR, Lim L, Phal PM, Rosenbaum JT. Orbital Inflammatory Disease. Semin Arthritis Rheum 2008;37:207-222
- ↑ Watkins LM, Carter KD, Nerad JA. Ocular Adnexal Lymphoma of the Extraocular Muscles: Case Series From the University of Iowa and Review of the Literature. Ophthal Plast Reconstr Surg. 2011;27:471-476
- ↑ Ahmad MS, Esmaeli B. Metastatic tumors of the orbit and ocular adnexa. Curr Opin Ophthalmol 2007;18:405-413
- ↑ Tarabishy AB, Schulte M, Papaliodis GN, Hoffman GS. Wegener’s Granulomatosis: Clinical Manifestations, Differential Diagnosis, and Management of Ocular and Systemic Disease. Surv Ophthalmol 2010;5:429-444
- ↑ Prabhakaran VC, Saeed P, Esmaeli B et al. Orbital and Adnexal Sarcoidosis. Arch Ophthalmol 2007;125(12):1657-1662
- ↑ Nageswaran S, Woods CR, Benjamin DK, Givner LB, Shetty AK. Orbital Cellulitis in Children. Pediatr Infect Dis J 2006;25:695-699
- ↑ Cockerham KP, Chan SS. Thyroid Eye Disease. Neurol Clin 2010;28:729-755
- ↑ Mannor GE, Rose GE, Moseley IF, et al. Outcome of orbital myositis: clinical features associated with recurrence. Ophthalmology 1997;104:409-414
- ↑ Green GA. Understanding NSAIDs: from aspiring to COX-2. Clinical cornerstone 2001;3:50-60
- ↑ 46.0 46.1 46.2 Rootman J, McCarthy M, White V, et al. Idiopathic sclerosing inflammation of the orbit: a distinct clinicopathological entity. Ophthalmology 1994;101:570-584
- ↑ 47.0 47.1 47.2 Garrity JA, Kennerdell JS, Johnson BL, et al. Cyclophosphamide in the treatment of orbital vasculitis. Am J OphthalmoI 1986;102:97-103
- ↑ 48.0 48.1 48.2 Lanciano R, Fowble B, Sergott RC, et al. The results of radiotherapy for orbital pseudotumor. Int J Radiat Oncol Biol Phys 1990;18:407-411
- ↑ 49.0 49.1 Orcutt J, Garner A, Henk J, Wright J. Treatment of idiopathic inflammatory orbital pseudotumors by radiotherapy. Br J Ophthalmol 1983;67:570-574
- ↑ Smitt MC, Donaldson SS. Radiation therapy for benign disease of the orbit. Semin Rad Oncol 1999;9:179-189
- ↑ Kennerdell J, Johnson B, Deutsch M. Radiation treatment of orbital lymphoid hyperplasia. Ophthalmology 1979;86:942-947
- ↑ Sergott R, Glaser J, Charyulu K. Radiotherapy for idiopathic inflammatory orbital pseudotumor: indications and results. Arch Ophthalmol 1981;99:8536
- ↑ 53.0 53.1 Hemady R, Tauber J, Foster CS. Immunosupressive drugs in immune and inflammatory ocular disease. Surv Ophthalmol. 1991;35:369-385
- ↑ Diaz-Llopez M, Menezo JL. Idiopathic inflammatory orbital pseudotumor and low-dose cyclosporine. Am J Ophthalmol 1989;107:547-548
- ↑ Zacharopoulos IP, Papadaki T, Manor RS, Briscoe D. Treatment of idiopathic orbital inflammatory disease with cyclosporine-A: a case presentation. Semin Ophthalmol 2009 2009;24:260-261
- ↑ 56.0 56.1 56.2 Chiu CS, Rubin PAD. Pharmacotherapies and Nonpharmacotherapies for Orbital Inflammatory Diseases. Int Ophthalmol Clin 2004;44(3):165-185
- ↑ Abeysiri P, Johnston NR, Molteno ACB. The Use of Topical Tacrolimus 0.1% Skin Ointment for Anterior Segment Conditions: A Case Series. Ophthalmology and Eye Diseases 2010;2:5-8
- ↑ Kymionis GD, Kankariya VP, Kontadakis GA. Tacrolimus ointment 0.03% for treatment of refractory childhood phlyctenular keratoconjunctivitis. Cornea 2012;31(8):950-952
- ↑ 59.0 59.1 59.2 59.3 Carruth BP, Wladis EJ. Inflammatory modulators and biologic agents in the treatment of idiopathic orbital inflammation. Curr Opin Ophthalmol 2012;23:420-426
- ↑ 60.0 60.1 60.2 Paris GL, Waltuch GF, Egbert PR. Treatment of refractory orbital psuedotumours with pulsed chemotherapy. Ophthal Plast Reconstr Surg 1990;6:96-101
- ↑ 61.0 61.1 Eagle K, King A, Fisher C, Souhami R. Cyclophosphamide induced remission in relapsed, progressive idiopathic orbital inflammation (PSeudotumor). Clin Oncol 1995;7:402-404
- ↑ Winn BJ, Rootman J. Sclerosing Orbital Inflammation and Systemic Disease. Ophthal Plast Reconstr Surg 2012;28(2):107-118
- ↑ 63.0 63.1 Lawson M, Vasilaras A, De Vries A, Mactaggart P, Nicol D. Urological implication of cyclophosphamide and ifosfamide. Scand J Urol Nephrol 2008;42(4):309-317
- ↑ 64.0 64.1 Gordon LK. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye 2006;20:1196-1206
- ↑ 65.0 65.1 Smith JR, Rosenbaum JT. A role for methotrexate in the management of non-infectious orbital inflammatory disease. Br J Ophthalmol 2001;85:1220-1224
- ↑ Shah SS, Lowder CY, Schmitt MA, et al. Low-dose methotrexate therapy for ocular inflammatory disease. Ophthalmology 1992;99:1419-1423
- ↑ Yue C, You X, Zhao L et al. The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int 2010;30(12):1553–1557
- ↑ Rudwaleit M, Claudepierre P, Wordsworth P et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 2009;36(4):801–808
- ↑ Philip J, Mease MD, Bernard S, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial The Lancet 2000;356(9227):385-390
- ↑ Brandt J, Kharioouzov A, Listing J, et al. Six-Month Results of a Double-Blind, Placebo-Controlled Trial of Etancercept Treatment in Patients With Active Ankylosing Spondylitis. Arthritis & Rheumatism 2003;48(6):1667-1675
- ↑ Baughman RP, Lower EE, Bradley DA, et al. Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest 2005;128:1062-1147
- ↑ Smith JA, Thompson DJS, Whitcup SM, et al. A randomized, placebo controlled, double masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum 2005;53:18-23
- ↑ Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med 2005;352:351-361.
- ↑ Ohno S, Nakamura S, Hori S, et al. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behcet's disease with refractory uveoretinitis. J Rheumatol 2004;31(7):1362-1368
- ↑ Capella MJ, Foster CS Long-term efficacy and safety of infliximab in the treatment of Behçet's disease. Ocular Immunology and Inflammation 2012;20(3):198-202
- ↑ Garrity JA, Coleman AW, Matteson EL, et al. Treatment of Recalcitrant Idiopathic Orbital Inflammation (Chronic Orbital Myositis) With Infliximab. Am J Ophthalmol 2004;138:925-930
- ↑ Miguel T, Abad S, Badelon I, Vignal C, et al. Successful treatment of idiopathic orbital inflammation with infliximab: An alternative to conventional steroid-sparing agents. Ophthal Plast Reconstr Surg 2008;24(5):415-417
- ↑ Sahlin S, Lignell B, Williams M, et al. Treatment of idiopathic sclerosing inflammation of the orbit (myositis) with infliximab. Acta Ophthalmol 2009;87:906-908
- ↑ Wilson MW, Shergy WJ, Haik BG. Infliximab in the Treatment of Recalcitrant Idiopathic Orbital Inflammation. 2004;20(5):381-399
- ↑ Ricart E, Panaccione R, Loftus EV, Tremaine WJ, Sandborn WJ. Infliximab for Crohn’s disease in clinical practice at the Mayo Clinic: the first 100 patients. Am J Gastroenterol 2001;96:722–729
- ↑ Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med 2004;350:2167–2179
- ↑ Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol 2002;20(Suppl 28):S152–S157
- ↑ On A, Hirschbien M, Williams H, Karesh J. CyberKnife radiosurgery and rituximab in the successful management of sclerosing idiopathic orbital inflammatory disease. Ophthalm Plas Reconstr Surg 2006;22:395-397
- ↑ Schafranski. Idiopathic orbital inflammatory disease successfully treated with rituximab. Clin Rheumatol 2009;28:225-226
- ↑ Ibrahim I, Barton A, Ibrahim A, Ho P. Idiopathic Orbital Inflammation Successfully treated Using Rituximab in a Patient with Rheumatoid Arthritis. J Rheumatol 2012;39(7):1485-1486
- ↑ Esmaeli B, Murray JL, Ahmadi MA, et al. Immunotherapy for low-grade non-Hodgkin secondary lymphoma of the orbit. Arch Ophthalmol 2002;120:1225–7
- ↑ Yokota S, Imagawa T, Mori M, et al. Efficacu and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 2008;371:998-1006
- ↑ Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized antiinterleuken-6 receptor antibody: a multicenter, double blind, placebo-controlled trial. Arthritis Rheum 2004;50:1761-1769
- ↑ de la Maza MS, Molina N, Gonzalez-Gonzalez, et al. Scleritis Therapy. Ophthalmology 2012;119:51-58
- ↑ Tappeiner C, Heinz C, Ganser G. Is Tocilizumab an Effective Option for Treatment of Refractory Uveitis Associated with Juvenile Idiopathic Arthritis? J Rheumatol 2012;39(6):1294-1295
- ↑ Venkiteshwaran A. Tocilizumab. MAbs 2009;1:432-8
- ↑ Oldfield V, Dhillon S, Plosker GL. Tocilizumab: a review of its use in the management of rheumatoid arthritis. Drugs 2009;69:609--32
- ↑ 93.0 93.1 93.2 Lim L, Suhler EB, Smith JR. Biologic therapies for inflammatory eye disease. Clin and Exper Ophthalmol 2006;34:365-374
- ↑ Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001;291:484–486
- ↑ Rosenbaum JT, George RK, Gordon C. The treatment of refractory uveitis with intravenous immunoglobulin. Am J Ophthalmol 1999;127:545–549.
- ↑ Shambal S, Lindner A, Zierz S. Successful treatment of orbital myositis with intravenous immunoglobulins. Muscle Nerve. 1998;21:1359-1360
- ↑ Symon Z, Schneebaum N, Eyal A, et al. Successful Intravenous Immunoglobulin Therapy for Resistant Inflammatory Pseudotumor of the Orbit. Thyroid 2005;14(4):398-399
- ↑ Baschieri L, Antonelli A, Nardi S, Alberti B, Lepri A, Canapicchi R, Fallahi. Intravenous immunoglobulin versus corticosteroid in treatment of Graves’ ophthalmopathy. Thyroid 1997;7:579–585.
- ↑ Pemberton JD, Fay A. Idiopathic Sclerosing Orbital Inflammation: A Review of Demographics, Clinical Presentation, Imaging, Pathology, Treatment, and Outcome. Ophthal Plast Reconstr Surg 2012;28:79-83