Oculopharyngeal Muscular Dystrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Oculopharyngeal muscular dystrophy (OPMD) is recognized by the following codes as per the standard nomenclature:
- International Classification of Diseases (ICD)
- Online Mendelian Inheritance of Man
Disease
OPMD is typically a late-onset genetic autosomal dominantly inherited disease of skeletal muscles. OPMD primarily involves the extra ocular muscles (oculo-) and the pharynx (-pharyngeal) but often involves the proximal muscles of the extremities as well. It is associated with progressive blepharoptosis (ptosis) and dysphagia. Other voluntary muscles may become affected over time. Muscle groups typically affected apart from eyelid elevator and extra ocular muscles include the proximal muscles of the lower limbs, and to a lesser extent, upper limbs as well. Symptom onset is typically in the 5th decade of life although in some cases can present earlier and more severely affected as well.
History of the disease
OPMD was originally reported in four members of a French-Canadian family in 1915,[1] and first described by M Victors, R Hayes, and RD Adams in 1962.[2]
Genetics
OPMD is due to a mutation of the pABPN1 gene, which was first described in 1988.[3] The genetic mutation results in an expansion of the alanine (GCN; denoted as such because four different codons – GCA, GCT, GCC and GCG – can encode the amino acid alanine) repeat region on chromosome 14q11.2.[3]This region is part of the polyadenylate-binding protein nuclear 1 gene (PABPN1, previous abbreviated as PABP2) N-terminus. PABN1 has regulatory functions in the polyadenylation of messenger RNA.[4] The N-terminal domain is normally (GCN)10 (denoted (GCN)10/Ala10) but the length of the alanine expansions range from +1 to +8.[5]The mutation is stable across generations and does not exhibit genetic anticipation.
Common mutations sorted by prevalence are:
- GCN)13, the most common dominant mutation in Quebec and worldwide
- (GCN)11, recessive polymorphism that is carried by 1-2% of North American, European and Japanese individuals. It causes a more severe phenotype when a patient is a compound heterozygote with both the (GCN)13 and the (GCN)11 variants
- Rare homozygote cases that carry two dominant mutations inherited from their two affected parents that have a much more severe phenotype
Although the inheritance pattern is typically autosomal dominant, an allelic autosomal recessive form has been documented (see common mutations above). In cases of autosomal dominant OPMD, while even a single mutation is sufficient to cause disease, occasionally, both alleles are mutated which results not only in an earlier manifestation of the disease but faster and more severe progression as well. In autosomal dominant patients every child of a heterozygous carrier has a 50% chance of inheriting the disease-causing mutation. There is nearly a complete penetrance pattern with an increased risk of presentation with increased age. Most individuals with the mutation have presented by the 7th decade of life (with average onset in the 5th decade). For those with an autosomal recessive mutation, the offspring will be obligate heterozygote (carriers) but their risk of being affected is less than 1% given the 2% carrier rate for the (GNC)11 recessive allele.[3]
Pathophysiology
The PABPN1 gene is responsible for the integrity and transport of mRNA in skeletal muscle cells through out the body, preserving its integrity and function. In patients with PABPN1 mutation, fragility of mRNA is increased resulting in clumps intracellularly. These clumps (intranuclear inclusions) result in progressive muscle cell dysfunction , eventual cell death and thus progressive clinical muscle dysfunction.
In addition to genetic testing, a muscle biopsy may be helpful to the diagnosis OPMD. The presence of intranuclear inclusions (INI) in myonuclei is a specific histologic hallmark of OPMD. Per slide, INI containing PABPN1, and many other RNA-binding proteins, can be seen by electron microscopy as tubular filaments in 4-5% of deltoid muscle nuclei of OPMD patients.[6] It is not known what is the cause of OPMD but the main hypotheses is that the pathology is caused by either 1) the clumping proteins (INI), or 2) the lack of the normal protein. Clinically unaffected muscles may also have histologic findings, such as rimmed vacuoles and ragged red fibers, that indicate a dystrophic pathology (but the findings in the unaffected muscles are not specific to OPMD).[7]
Epidemiology
The largest cluster of OPMD is in Québec, Canada, but cases have been observed in over 30 countries.[8] Québec’s French-Canadian population has an OPMD frequency of 1/1000,[9] while Israel’s Burkhara Jewish population have the world’s highest gene frequency with 1/600 affected.[10]
Diagnosis
History
Patients typically have a slowly progressive bilateral, albeit often asymmetric, blepharoptosis. Patients sometimes compensate by adopting a “chin up” position with neck extension in order to displace their visual axis inferior to the ptotic upper eyelids. Dysphagia begins with solid foods but may progress to include liquids. Difficulty with speech, or a hoarse weak voice, may be an prominent feature. Some patients will notice progressive fatigue and a slowing or change of gait. Lower limb weakness is more common than weakness in the upper limbs. Proximal upper limb weakness is mostly noted in the deltoid muscles, if at all.
Physical Examination
The clinical signs of OPMD will vary with age, though all patients will ultimately develop ptosis and dysphagia with some degree of proximal leg weakness. Depending on the genetic group involved, signs include: [11][12]
| Finding | Incidence | Key features |
|---|---|---|
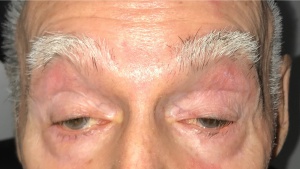
| Blepharoptosis
(Figure 1) |
92-100% |
|
| Dysphagia | 40-100% |
|
| Dysarthrophonia | 25-75% |
|
| Proximal muscle weakness | 20-81% |
|
| Facial weakness | 18-81% |
|
| Ocular motility disorder | 6-68% |
|
Pertinent normal findings are unimpaired intrinsic eye muscles and normal retinal function.
Clinical Diagnosis
The following three criteria are helpful in establishing the diagnosis of OPMD on clinical grounds:[9]
- Positive family history with involvement of two or more generations (important side note: in any patient with a suspected genetic disorder, it is important to complete a pedigree)
- Presence of ptosis or previous corrected surgery for ptosis (one or both palpebral fissures smaller than or equal to 8mm (he average is 10 mm vertically).
- Presence of dysphagia (inability to drink 80cc of cold water in less than 8 seconds)
The above criteria are specific for penetrance of the (GCN)13 mutation in 31% patients in their 50s, while it is 99% specific for those over the age of 69 years.[15]
Laboratory test
Sensitivity and specificity of a single polymerase chain reaction (PCR) testing for the PABPN1 mutation to determine carrier status of an individual is greater than 99%. The size of the DNA products can be used to determine the number the number of GCN insertions and the exact GCN sequence. Indications for DNA testing of a symptomatic individual are:[12]
- Confirmation of the diagnosis in a family never tested
- Confirmation of a clinical picture that presents a diagnostic dilemma
- Evaluation of the size of the mutation as a possible indicator of severity
- Evaluation of a compound heterozygote status in a patient with a severe earlier-onset form
- Confirming those who may suffer from recessive OPMD
Imaging with magnetic resonance imaging (MRI) may play a role in identifying fatty degeneration of extra ocular muscles, although it may be both a late and non-specific radiologic feature [16]
Differential diagnosis
- Ocular myasthenia gravis (unlike OPMD, ptosis is fluctuating and fatigable)
- Mitochondrial myopathy
- Chronic progressive external ophthalmoplegia (CPEO; Complete external ophthalmoplegia is rare in OPMD)
- Mitochondrial neurogastrointestinal encephalopathy syndrome (MNGIE; Sometimes referred to as pediatric OPMD, but no PABPN1 (GCN)n mutations have been found)
- Oculopharygnodistal myopathy (OPDM; weakness appears earlier in life. Facial diplegia is seen, while this is not a dramatic feature in OPMD)
- Myotonic dystrophy (unlike OPMD, myotonia, the inability to relax muscles after use, is common.) Extraocular muscle deviations often produce diplopia. Also, myotonic dystrophy ocular findings include “Christmas Tree” cataract changes, and there is a relatively low intraocular pressure in ocular myotonic dystrophy, possibly due to ciliary body detachment. (Ptosis is common to both)[17]
- Polymyositis (will not have ptosis)
- Inclusion body myositis (IBM; including myositis, polymyositis and progressive bulbar palsy. These do not have ptosis)
- Autosomal dominant distal myopathy (vocal cord weakness without ptosis)
- Congenital fibrosis of the extraocular muscles (CFEOM; congenital ptosis without dysphagia).
Management
Systemic management
Currently there is no established medical treatment for OPMD. A high-protein diet, to limit weight loss, and non-strenuous cardiovascular exercises, to limit muscle breakdown, should be promoted.[12]Ambulation assistance with a cane or wheelchair may become necessary.
Symptomatic dysphagia should be evaluated. Severe dysphagia, marked weight loss, near-fatal choking or recurrent pneumonia warrant urgent evaluation. Cricopharyngeal myotomy (upper esophageal sphincter myectomy) will alleviate symptoms in most cases.[18] Cricopharyngeal dilation is the most used treatment, though scientific evidence is still lacking to support its long term benefits. Cricopharyngeal botulinum toxin, autologous myoblast transplantation after myectomy, and use of the molecule trehalose to ameliorate the OPMD phenotype are among the therapies currently under investigation in humans.[19] [20][21][22] Animal trials to silence the mutant PABPN1 gene and replace it with a functional copy have been successful in mice, and efforts are underway to evaluate if this success is transferrable to human patients.[23]
Blepharoptosis management
Blepharoptosis correction is recommended when the eyelids interfere with vision, strain or the patient complains of cervical pain due to constant extension of the neck. Both resection of the levator palpebrae aponeurosis and frontalis suspension are possible.[24] Originally, it was thought that Beard’s congenital ptosis guidelines could be applied to those with OPMD,[25] and that levator palpebrae aponeurosis resection would a reasonable primary procedure based on those guidelines.[26] However, unlike congenital ptosis, which is a static disease, OPMD progressively worsens with time. Therefore, Beard’s guidelines tend to yield high rates of reoperation in those with OPMD who undergo levator advancement.
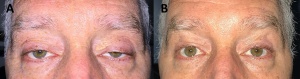
For this reason, many surgeons now opt for a primary frontalis suspension, even if levator function is >4mm.[27]Frontalis suspension may also be used in reoperations for patients that have had prior levator resection. Frontalis suspension has been shown to achieve adequate results, including in those with good levator function.[28] Given a multitude of publications with differing suspension materials, suspension patterns and follow-up time, no one technique or material is considered the gold-standard. However, synthetic materials have gained in popularity with large studies on Polypropylene sutures and silicone bands yielding acceptable results.[28][29] (Figure 2)
Yet another unconventional surgical management for OPMD and other forms of myopathic ptosis is the tarsoconjunctival switch flap technique where a full thickness upper eyelid shortening including levator resection is switched as a graft to the lower eyelid raising the lower eyelid margin resulting in a vertical shift of the palpebral fissure without increasing lagophthalmos.
Contraindications to blepharoplasty are marked ophthalmoplegia, dry eye syndrome, or poor orbicularis function.[12]
Blepharoptosis complications
Lagophthalmos is the most significant consideration in the management of OPMD. When performing a frontalis suspension under local anesthesia, aiming for a margin reflex distance-1 (MRD1) greater than 3mm, especially in patients with poor orbicularis tone, can yield significant corneal exposure. Aiming for a conservative MRD-1 (typically ≤2.5mm) will minimize lagophthalmos and potentially serious adverse events. Sling exposure, infection or blepharoptosis recurrence due to disease progression, knot slippage or material cheese-wiring are all possible. Polypropylene and silicone bands can, in most cases, be adjusted as a secondary procedure to modify MRD-1.
Prognosis
OPMD is a progressive disease. Patients will experience progressive loss of muscle strength. Repeat blepharoptosis surgeries may be minimized by considering primary frontalis suspension, especially in cases with reduced levator function. With adequate treatment of pharyngeal dysfunction and nutrition, life expectancy is not shortened.[13] . Newer modalities such as genetic testing with gene therapy seems to show promise in mouse models.[23]
References
- ↑ Taylor, EW. Progressive vagus—glossopharyngeal paralysis with ptosis: a contribution to the group of family diseases. J. Nerv Ment Dis. 1915 Mar; 42(3) 129-139
- ↑ Victor M, Hayes R, Adams RD. Oculopharyngeal muscular dystrophy. A familial disease of late life characterized by dysphagia and progressive ptosis of the eyelids. N Engl J Med. 1962 Dec 20;267:1267-72
- ↑ 3.0 3.1 3.2 Brais B, Bouchard JP, Xie YG, Rochefort DL, Chrétien N, Tomé FM, Lafrenière RG, Rommens JM, Uyama E, Nohira O, Blumen S, Korczyn AD, Heutink P, Mathieu J, Duranceau A, Codère F, Fardeau M, Rouleau GA. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998 Feb;18(2):164-7.
- ↑ Keller RW, Kühn U, Aragón M, Bornikova L, Wahle E, Bear DG. The nuclear poly(A) binding protein, PABP2, forms an oligomeric particle covering the length of the poly(A) tail. J Mol Biol. 2000 Mar 31;297(3):569-83
- ↑ Raz V, Butler-Browne G, van Engelen B, Brais B. 191st ENMC international workshop: recent advances in oculopharyngeal muscular dystrophy research: from bench to bedside 8-10 June 2012, Naarden, The Netherlands. Neuromuscul Disord.2013 Jun;23(6):516-23
- ↑ Tomé FM, Fardeau M. Nuclear inclusions in oculopharyngeal dystrophy. Acta Neuropathol. 1980;49(1):85-7
- ↑ Gidaro T, Negroni E, Perié S, Mirabella M, Lainé J, Lacau St Guily J, Butler-Browne G, Mouly V, Trollet C. Atrophy, fibrosis, and increased PAX7-positive cells in pharyngeal muscles of oculopharyngeal muscular dystrophy patients. J Neuropathol Exp Neurol. 2013 Mar;72(3):234-43
- ↑ Brais B. Oculopharyngeal muscular dystrophy: a late-onset polyalanine disease. Cytogenet Genome Res. 2003;100(1-4):252-60
- ↑ 9.0 9.1 Brais B, Xie YG, Sanson M, Morgan K, Weissenbach J, Korczyn AD, Blumen SC, Fardeau M, Tomé FM, Bouchard JP, et al. The oculopharyngeal muscular dystrophy locus maps to the region of the cardiac alpha and beta myosin heavy chain genes on chromosome 14q11.2-q13. Hum Mol Genet. 1995 Mar;4(3):429-34
- ↑ Blumen SC, Nisipeanu P, Sadeh M, Asherov A, Blumen N, Wirguin Y, Khilkevich O, Carasso RL, Korczyn AD. Epidemiology and inheritance of oculopharyngeal muscular dystrophy in Israel. Neuromuscul Disord. 1997 Oct;7 Suppl 1:S38-40
- ↑ Rüegg S, Lehky Hagen M, Hohl U, Kappos L, Fuhr P, Plasilov M, Müller H, Heinimann K. Oculopharyngeal muscular dystrophy - an under-diagnosed disorder? Swiss Med Wkly. 2005 Oct 1;135(39-40):574-86
- ↑ 12.0 12.1 12.2 12.3 Brais B. Oculopharyngeal muscular dystrophy. Handb Clin Neurol. 2011;101:181-92
- ↑ 13.0 13.1 Becher MW, Morrison L, Davis LE, Maki WC, King MK, Bicknell JM, Reinert BL, Bartolo C, Bear DG. Oculopharyngeal muscular dystrophy in Hispanic New Mexicans. JAMA. 2001 Nov 21;286(19):2437-40
- ↑ 14.0 14.1 Bouchard JP. André Barbeau and the oculopharyngeal muscular dystrophy in French Canada and North America. Neuromuscul Disord. 1997 Oct;7 Suppl 1:S5-11
- ↑ Brais B, Bouchard JP, Gosselin F, Xie YG, Fardeau M, Tomé FM, Rouleau GA. Using the full power of linkage analysis in 11 French Canadian families to fine map the oculopharyngeal muscular dystrophy gene. Neuromuscul Disord. 1997 Oct;7 Suppl 1:S70-4
- ↑ Lassche S, Küsters B, Heerschap A, Schyns MVP, Ottenheijm CAC, Voermans NC, van Engelen BGM. Correlation Between Quantitative MRI and Muscle Histopathology in Muscle Biopsies from Healthy Controls and Patients with IBM, FSHD and OPMD. J Neuromuscul Dis. 2020;7(4):495-504. doi: 10.3233/JND-200543. PMID: 32925090; PMCID: PMC7739972.).
- ↑ Ocular_Manifestations_of_Myotonic_Dystrophy https://eyewiki.aao.org/Ocular_Manifestations_of_Myotonic_Dystrophy
- ↑ Duranceau A, Forand MD, Fauteux JP. Surgery in oculopharyngeal muscular dystrophy. Am J Surg. 1980 Jan;139(1):33-9
- ↑ Youssof S, Schrader RM, Romero-Clark C, Roy G, Spafford M. Safety of botulinum toxin for dysphagia in oculopharyngeal muscular dystrophy. Muscle Nerve. 2014 Apr;49(4):601-3
- ↑ Manjaly JG, Vaughan-Shaw PG, Dale OT, Tyler S, Corlett JC, Frost RA. Cricopharyngeal dilatation for the long-term treatment of dysphagia in oculopharyngeal muscular dystrophy. Dysphagia. 2012 Jun;27(2):216-20
- ↑ Périé S, Trollet C, Mouly V, Vanneaux V, Mamchaoui K, Bouazza B, Marolleau JP, Laforêt P, Chapon F, Eymard B, Butler-Browne G, Larghero J, St Guily JL. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol Ther. 2014 Jan;22(1):219-25
- ↑ Safety tolerability and efficacy study of cabaletta to treat oculopharyngeal muscular dystrophy (OPMD) patients (HOPEMD). (2013). Identification No. NCT02015481
- ↑ 23.0 23.1 Malerba A, Klein P, Bachtarzi H, Jarmin SA, Cordova G, Ferry A, Strings V, Espinoza MP, Mamchaoui K, Blumen SC, St Guily JL, Mouly V, Graham M, Butler-Browne G, Suhy DA, Trollet C, Dickson G. PABPN1 gene therapy for oculopharyngeal muscular dystrophy. Nat Commun. 2017 Mar 31;8:14848. doi: 10.1038/ncomms14848. PMID: 28361972; PMCID: PMC5380963.
- ↑ Codère F. Oculopharyngeal muscular dystrophy. Can J Ophthalmol. 1993 Feb;28(1):1-2
- ↑ Molgat YM, Rodrigue D. Correction of blepharoptosis in oculopharyngeal muscular dystrophy: review of 91 cases. Can J Ophthalmol. 1993 Feb;28(1):11-4
- ↑ Rodrigue D, Molgat YM. Surgical correction of blepharoptosis in oculopharyngeal muscular dystrophy. Neuromuscul Disord. 1997 Oct;7 Suppl 1:S82-4
- ↑ Allen RC, Jaramillo J, Black R, Sandoval D, Morrison L, Qualls C, Carter KD, Nerad JA. Clinical characterization and blepharoptosis surgery outcomes in Hispanic New Mexicans with oculopharyngeal muscular dystrophy. Ophthalmic Plast Reconstr Surg. 2009 Mar-Apr;25(2):103-8
- ↑ 28.0 28.1 Allen RC, Zimmerman MB, Watterberg EA, Morrison LA, Carter KD. Primary bilateral silicone frontalis suspension for good levator function ptosis in oculopharyngeal muscular dystrophy. Br J Ophthalmol. 2012 Jun;96(6):841-5
- ↑ Kalin-Hajdu E, Attas-Fox L, Huang X, Hardy I, Codère F. Comparison of Two Polypropylene Frontalis Suspension Techniques in 92 Patients With Oculopharyngeal Muscular Dystrophy. Ophthalmic Plast Reconstr Surg. 2017 Jan/Feb;33(1):57-60.