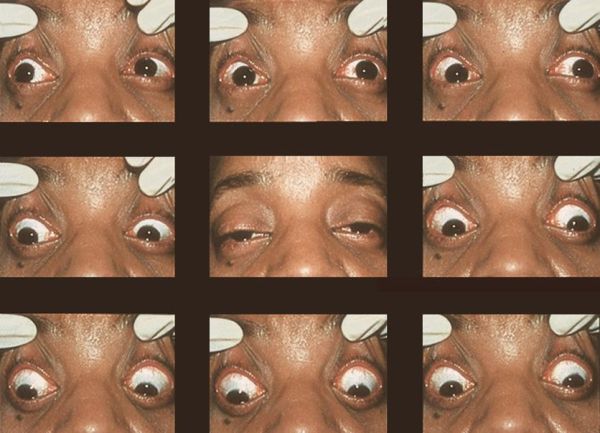
Chronic Progressive External Ophthalmoplegia (CPEO)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Chronic progressive external ophthalmoplegia (CPEO) describes an array of hereditary myopathies affecting extraocular muscles (EOMs), commonly manifesting as bilateral progressive and constant ptosis and decreased motility of eye muscles not-following a cranial nerve palsy without pupil involvement. [1][2] As the name suggests, it is a chronic, progressive, bilateral, typically symmetric, and external (i.e., spares the pupil) ophthalmoplegia. CPEO is associated with mitochondrial disease and can occur as isolated oculomotor symptoms (isolated CPEO) or in conjunction with other systemic findings (“CPEO-plus”). Depending on additional clinical features, CPEO can be evaluated as part of a systemic myopathic or neurologic syndrome.[1]
Ptosis and ophthalmoplegia are nonspecific presentations however and can be mimicked by other neurogenic, neuromuscular junction, and myogenic etiologies. CPEO can sometimes difficult to diagnose however especially when the patient is asymmetric, lacks other systemic signs, or has no family history of CPEO. Thus, it is crucial for physicians to have CPEO in their differential diagnoses to avoid unnecessary tests or delays in diagnosis.[3]
There are cases, although rare, that can present without ptosis in the adult life, making the diagnosis challenging[4]
Pathogenesis
Up to 60% cases of mitochondrial CPEO are due to mitochondrial DNA (mtDNA) deletions (ranging from 1.3 to 1.9 kb).[1] Other cases however are due to nuclear DNA (nDNA)-related defects of mtDNA maintenance (e.g., POLG1, ANT, C10orf2/twinkle or POLG2).[5] Sporadic cases of CPEO suggest de novo mutations in mtDNA while autosomal dominant or recessive inheritance patterns point to nDNA mutations.[6]
Enzymes encoded by mtDNA play a key role in oxidative phosphorylation necessary to meet the metabolic demands of active muscle.[7] To maintain a high fatigue resistance, EOMs have adapted to have both a higher mitochondrial content and higher metabolic demand compared to skeletal muscles. These properties are hypothesized to be one of the reasons why EOMs are especially vulnerable to the oxidative phosphorylation dysfunction that occurs in CPEO.[7]
There are more mutations known both for nuclear DNA and mtDNA . The single mtDNA deletions are most commonly associated to CPEO, there are at least 35 different mtDNA point mutations and other variant mutations( 12 )in DNA genes that can manifest with CPEO isolated.[2][2]
Clinical Diagnosis
Patients with CPEO typically present with bilateral, symmetric, painless, and pupil-spared ptosis and ophthalmoplegia. The disease most commonly begins in the third or fourth decade of life.[1] [3] As the name implies, CPEO takes a slowly progressive course, over a course of years, making it distinct from other etiologies that cause acute/subacute or static forms of ophthalmoplegia.[1][3][8] With bilateral and symmetrical involvement of the eyes, patients can be asymptomatic without diplopia even with severe defects in ocular motility.[1] They will also compensate for their lack of ocular motility by moving their head.[5] In addition, due to the gradual course, patients may not notice the eyelid droop unless it is brought up by a third party.[3] Other less common ophthalmic manifestations include pigmentary retinopathy and optic atrophy.[1][3][5][6]. Again, there are cases that may present without ptosis or asymmetric.
Pain, proptosis, periorbital swelling, lid lag/retraction and pupil involvement are not symptoms of CPEO and indicate a different etiology. Unilateral or rapidly progressive symptoms are also atypical and should prompt additional evaluation including neuroimaging.[3] Detailed family history is important in identifying possible inherited conditions.
Other non-ophthalmic manifestations include sensorineural hearing loss and dysphagia.[9]
If CPEO is associated to another syndrome, it is considered a CPEO-plus syndrome. The following sections describe the additional clinical symptoms and signs of these pathologies.
Differential Diagnosis
Kearns-Sayre Syndrome
Unlike isolated CPEO that commonly presents at the third or fourth decade of life, patients with KSS typically have symptom onset before age 20.[10] Patients with KSS also have an interesting bilateral pigmentary retinopathy, a degenerative condition of the retina that impairs function of pigment epithelial cells and photoreceptors. Patients experience a progressive loss of peripheral and night vision. Thus, a dilated fundus exam should be performed in cases of CPEO to visualize any areas of hypo- or hyper-pigmentation indicating retinal pigment epithelium (RPE) disturbances that might suggest KSS.[3] The atypical RPE depigmentation has been described as having a “salt-and-pepper” or “moth-eaten” appearance.[1] Other associated findings in KSS include cardiovascular conduction defects, elevated protein in the cerebrospinal fluid (CSF), and cerebellar ataxia. Patients with KSS should therefore be considered for cardiac assessment, a lumbar puncture, and a thorough neurologic assessment.[1][10]
Oculopharyngeal Musculodystrophy
Oculopharyngeal muscular dystrophy (OPMD) is an autosomal dominant disorder with pathologic GCG trinucleotide repeat expansions in the polyalanine-binding protein 1 (PABP1) gene.[11] The mutated PAPB1 proteins aggregate as intranuclear tubular filaments and can cause failure of muscle regeneration through an unclear mechanism.[11] Unlike some other CPEO-plus syndromes, OPMD is not a mitochondrial myopathy. This disorder is most prevalent among people of French-Canadian origin. Symptoms present at the fifth decade of life and in addition to CPEO, include bulbar symptoms such as dysphagia (pharyngeal muscle weakness), weakness of the orbicularis oculi muscle, and proximal limb weakness.[1][3]
Myotonic Dystrophy
Myotonic dystrophy comes in congenital, childhood, and classical forms, with onset at birth, childhood, and adulthood, respectively.[12] This syndrome can manifest as a multitude of both ocular and systemic findings. Ocular symptoms include CPEO, lid lag, slow saccades, and cataracts. Distal muscle weakness is common, with patients reporting difficulties with activities requiring fine motor control of hands.[1][12] Myotonia, or delayed relaxation of muscles following contraction, most consistently involves the hand muscles, which can be observed as a “percussion myotonia” during the physical exam when the thenar muscle is percussed by a reflex hammer.[13] Myotonia of the facial muscles, tongue, and other bulbar muscles results in impairment of facial expressions, chewing, talking, and swallowing.[13]
Congenital Fibrosis of Extraocular Muscles
Congenital Fibrosis of the Extraocular Muscles (CFEOM) is a severe form of strabismus with deficits in ocular motility. Patients with this congenital, non-progressive disorder have restrictive ophthalmoplegia and eye misalignment, with severe congenital ptosis and a resulting prominent chin-up head position. Deficits of vertical eye movements, especially upgaze, are a hallmark of the condition, and patients’ eyes are often stuck in infraduction. The horizontal eye movement deficits are more variable, ranging from full horizontal motility to nearly complete ophthalmoplegia. Eye alignment in primary position can be exotropic, esotropic, or straight
Three forms
- CFEOM1= autosomal dominant, bilateral ptosis, deficient elevation (frequently 20-30 degrees infraducted), and restricted horizontal movements compensatory chin-up head position. Findings symmetric no other neurologic deficits. Other: none.
- CFEOM2= autosomal recessive, bilateral ophthalmoplegia and ptosis. Frequently exotropic and vertically midline. Restriction of both horizontal and vertical eye movements. Other: small, sluggish pupils, low visual acuity consistent with retinal dysfunction.
- CFEOM3= autosomal dominant, Isolated or Syndromic, variable phenotype, from mild to quite severe, and can range even within the same family. Unilateral or asymmetric involvement, +/-ptosis, mil ability to elevate eye(s). Deficits of horizontal motility are also quite variable. As detailed below, CFEOM3 can also be associated with a variety of other neurological abnormalities, such as associated facial palsy or weakness. Other: Isolated none; Syndromic: cranial and spinal peripheral neuropathies, developmental delay, intellectual and social disability, and brain malformations; facial weakness and vocal cord paralysis; facial dysmorphisms, Kallmann syndrome (anosmia with hypogonadal hypogonadism), and can develop an axonal peripheral neuropathy and cyclic vomiting; There are mutation-specific correlations with specific brain malformations including thin-to-absent anterior commissure and corpus callosum, dysmorphic basal ganglia, brainstem hypoplasia, and hypoplastic or absent olfactory sulci, olfactory bulbs, and facial nerves.
Other
Other syndromes (usually in childhood or before 20years of age) include Pearson syndrome, Alpers syndrome, Leigh syndorme, Sensory ataxic neuropathy, dysarthria, ophthalmoparesis; Mitochondrial encephalomyopathy, lactic acidosis and stroke like episodes(MELAS), and Mitochondrial neurogastrointestinal encephalomyopathy[15]
Physical Exam
A comprehensive physical exam, with focus on the ophthalmologic and neurological components, is crucial in identifying distinguishing phenotypes of CPEO and its associated syndromes. The severity of the ophthalmoplegia can be quantified by measuring the uniocular fields of fixation and ductions. The Goldmann perimeter can be used to map the range of extraocular movement (EOM) in the various fields of gaze. For example, in the right eye, the cardinal axes are 0o (lateral rectus), 67o (superior rectus), 141o (inferior oblique), 180o (medical rectus), 216o (superior oblique), and 293o (inferior rectus). During the exam, one eye is occluded and the patient follows an illuminated target along each of the axes until central fixation on the target is lost. In one study, overall mean range of EOM was decreased by 73% in patients with CPEO compared to controls.[16][17] The degree of ptosis can be measured by the vertical fissure height (VFH), levator function (LF), and margin-reflex distance (MRD).[17]
Laboratory Studies
While CPEO is a clinical diagnosis, laboratory studies can aid in confirming the diagnosis, as well as ruling out alternate diagnoses. Serum lactate and creatinine kinase and CSF lactate levels may be elevated in CPEO, but this finding is neither sensitive or specific.[1][9] Absence of anti-acetylcholinesterase antibodies and thyroid autoantibodies can help in the evaluation for myasthenia gravis and thyroid-associated ophthalmopathy, respectively, when history alone is not sufficient.[3]
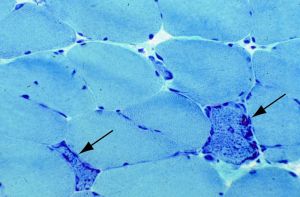
Characteristic findings on muscle biopsy can also distinguish between different underlying myopathies. KSS will show cytochrome c oxidase negative fibers with ragged red fibers on Gomori’s trichome stain.[10]
Oculopharyngeal muscular dystrophy reveal basophilic rimmed vacuoles and filamentous intranuclear inclusions.[11] Muscle biopsies of patients with myotonic dystrophy exhibits internal nuclei, ring fibers, sarcoplasmic masses, and early type I fiber atrophy.[13] If clinical or family history point to a specific syndrome, genetic testing may be invaluable in determining prognosis and additional studies that need to be done. An experienced laboratory may be necessary in conducting the genetic analysis given the extensive list of potential nuclear and mitochondrial DNA involved.
Sequencing/Genetic testing to confirm diagnosis may include mtDNA
nDNA mutation points: SLC25A4, TWNK, POLG2, SPG7, DNA2, RNASEH1, TOP3A, TK2, DGUOK, RRM2B, and GMPR
Imaging
Orbital magnetic resonance imaging (MRI) often reveals atrophy of EOMs in CPEO patients. One study showed a 43% decrease in the cross-sectional area of EOMs in CPEO patients compared to controls.[16][18]Brain MRI findings are numerous and nonspecific, including white matter hyperintensity, cortical atrophy, cerebellar atrophy, and brainstem hyperintensity.[19] Optic Coherence Tomography may show reduced thickness of the outer retinal layer and macular central fovea as well as reduced volume of the optic nerve head and rim.[20]
Differential Diagnosis
- Myasthenia gravis
- Thyroid-associated ophthalmopathy
- Congenital fibrosis of extra-ocular muscles
- Ocular sarcoidosis
- Botulism
- CN 3 palsy
Management
While there is no definitive cure for CPEO or its associated syndromes, control of symptoms can markedly improve the patient’s quality of life. Referral for management of concomitant neurologic or cardiac disease is indicated. Approximately one-third of patients experience constant or intermittent diplopia.[3] Prism lenses are effective in controlling diplopia after a full orthoptic assessment of direction and magnitude of eye deviations. The prism compensates for the deviated eye(s) by refracting the light so that the image falls onto the macula of each eye. While some patients opt for surgical correction of horizontal recti muscles, diplopia or strabismus may reoccur over time due to the progressive nature of CPEO.[3]
Clinically significant ptosis can be corrected surgically. If levator function remains moderate or good, advancement or resection of the levator palpebrae superioris may be considered to maximize the muscle’s elevation of the lid. However, typically levator function will progressively worsen resulting in minimal levator excursion. In cases of poor levator function, eyelid suspension to the frontalis muscle with autogenous or synthetic sling material should be considered.[3][21] Careful pre-operative evaluation and surgery by a trained oculoplastics surgeon is vital. Overcorrection of ptosis with surgery can result in lagophthalmos and corneal exposure, leading to potentially blinding complications of exposure keratopathy, corneal ulcers, or ocular perforation.[1][21]. Some authors have used a frontalis sling with silicone rod to correct ptosis in CPEO[22]
Since keratopathy is one of the most frequent complications of CPEO, fixing exposure is key. There is a new proposed way to surgically treat CPEO with palpebral fissure transfer (PFT) with lower eyelid elevation with no spacer to successfully cover the cornea [23]
Additional Resources
University of Utah Moran Eye Institute Videos CPEO1, CPEO2
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Lee, A. G. & Brazis, P. W. Chronic progressive external ophthalmoplegia. Curr. Neurol. Neurosci. Rep. 2, 413–417 (2002).
- ↑ 2.0 2.1 2.2 Hirano M, Pitceathly RDS. Progressive external ophthalmoplegia. Handb Clin Neurol. 2023;194:9-21. doi:10.1016/B978-0-12-821751-1.00018-X
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Yu-Wai-Man, P. Chronic Progressive External Ophthalmoplegia Secondary to Nuclear-Encoded Mitochondrial Genes. in Mitochondrial Case Studies 159–169 (Elsevier, 2016).
- ↑ Bucelli, Robert C. MD, PhD; Lee, Michael S. MD; McClelland, Collin M. MD Chronic Progressive External Ophthalmoplegia in the Absence of Ptosis, Journal of Neuro-Ophthalmology: September 2016 - Volume 36 - Issue 3 - p 270-274 doi: 10.1097/WNO.0000000000000384
- ↑ 5.0 5.1 5.2 Tarnopolsky, M. Chronic Progressive External Ophthalmoplegia (CPEO). in Mitochondrial Case Studies 49–53 (Elsevier, 2016).
- ↑ 6.0 6.1 DiMauro, S., Schon, E. A., Carelli, V. & Hirano, M. The clinical maze of mitochondrial neurology. Nat. Rev. Neurol. 9, 429–444 (2013).
- ↑ 7.0 7.1 Yu Wai Man, C. Y., Chinnery, P. F. & Griffiths, P. G. Extraocular muscles have fundamentally distinct properties that make them selectively vulnerable to certain disorders. Neuromuscul. Disord. 15, 17–23 (2005).
- ↑ Ahmad, S. & Ghani, S. Kearns-Sayre syndrome: An unusual ophthalmic presentation. Oman J. Ophthalmol. 5, 115 (2012).
- ↑ 9.0 9.1 Heighton, J. N., Brady, L. I., Newman, M. C. & Tarnopolsky, M. A. Clinical and demographic features of chronic progressive external ophthalmoplegia in a large adult-onset cohort. Mitochondrion 44, 15–19 (2019).
- ↑ 10.0 10.1 10.2 Moraes, C. T. et al. Mitochondrial DNA Deletions in Progressive External Ophthalmoplegia and Kearns-Sayre Syndrome. N. Engl. J. Med. 320,1293–1299 (1989).
- ↑ 11.0 11.1 11.2 Hill, M. E. et al. Oculopharyngeal muscular dystrophy Phenotypic and genotypic studies in a UK population. Brain 124, 522–526 (2001).
- ↑ 12.0 12.1 Machuca‐Tzili, L., Brook, D. & Hilton‐Jones, D. Clinical and molecular aspects of the myotonic dystrophies: A review. Muscle Nerve 32, 1–18 (2005).
- ↑ 13.0 13.1 13.2 Turner, C. & Hilton-Jones, D. The myotonic dystrophies: diagnosis and management. J. Neurol. Neurosurg. Psychiatry 81, 358–367 (2010).
- ↑ Whitman MC, Hunter DG. Clinical Education / Education / Disease Reviews OCT 14, 2015 Congenital Fibrosis of the Extraocular Muscles (CFEOM) DOI: https://www.aao.org/disease-review/neuro-ophthalmology-congenital-fibrosis-of-extraoc
- ↑ Bucelli, Robert C. MD, PhD; Lee, Michael S. MD; McClelland, Collin M. MD Chronic Progressive External Ophthalmoplegia in the Absence of Ptosis, Journal of Neuro-Ophthalmology: September 2016 - Volume 36 - Issue 3 - p 270-274 doi: 10.1097/WNO.0000000000000384
- ↑ 16.0 16.1 Pitceathly, R. D. S. et al. Extra-ocular muscle MRI in genetically-defined mitochondrial disease. Eur. Radiol. 26, 130–137 (2016).
- ↑ 17.0 17.1 Man, C. Y. W., Smith, T., Chinnery, P. F., Turnbull, D. M. & Griffiths, P. G. Assessment of visual function in chronic progressive external ophthalmoplegia. Eye 20, 564 (2006).
- ↑ Carlow, T. J., Depper, M. H. & Orrison, W. W. MR of extraocular muscles in chronic progressive external ophthalmoplegia. Am. J. Neuroradiol. 19, 95–99 (1998).
- ↑ Barragán-Campos, H. M. et al. Brain Magnetic Resonance Imaging Findings in Patients With Mitochondrial Cytopathies. Arch. Neurol. 62, 737–742 (2005).
- ↑ Wu, Y., Kang, L., Wu, H.-L., Hou, Y. & Wang, Z.-X. Optical coherence tomography findings in chronic progressive external ophthalmoplegia: Chin. Med. J. (Engl.) 132, 1202–1207 (2019).
- ↑ 21.0 21.1 Takahashi, Y., Leibovitch, I. & Kakizaki, H. Frontalis Suspension Surgery in Upper Eyelid Blepharoptosis. Open Ophthalmol. J. 4, 91–97 (2010).
- ↑ Ahn J, Kim NJ, Choung HK, et alFrontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegiaBritish Journal of Ophthalmology 2008;92:1685-1688.?
- ↑ Karimi N, Kashkouli MB, Tahanian F, Abdolalizadeh P, Jafarpour S, Ghahvehchian H. Long-term Results of Palpebral Fissure Transfer With No Lower Eyelid Spacer in Chronic Progressive External Ophthalmoplegia. Am J Ophthalmol. 2021 Jul 30;234:99-107. doi: 10.1016/j.ajo.2021.07.027. Epub ahead of print. PMID: 34339660.