Inclusion Body Myositis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
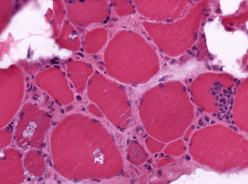
Inclusion Body Myositis (Sporadic Inclusion Body Myositis)
Disease
Sporadic inclusion body myositis (IBM) is a slowly progressive myopathic process that generally affects individuals more than 50 years of age and does not respond to immunosuppressive therapy. The typical pattern of muscle involvement includes prominent and often asymmetric weakness of the triceps, wrist flexors, distal finger flexors, quadriceps, and ankle dorsiflexors. Dysphagia is a common and occasionally presenting feature. Facial weakness, particularly orbicularis oculi weakness, is less common but can be seen with careful neuromuscular exam[1][2]IBM has been linked to cardiovascular and other autoimmune diseases.[3]
Epidemiology
The prevalence of IBM ranges from 4.9-10.7 per million and depends on geographic area, ethnicity, sex, and age. Due to the high rate of misdiagnosis and the average time of diagnosis being 5.2 years, the latest prevalence figure may be an underestimate. Symptom onset before age 60 occurs in 18 to 20 percent of patients. Unlike many other autoimmune diseases, this disease affects men more often than women with a reported male to female ratio of 3:1.[4]
Etiology
The underlying cause of IBM is unknown. Autoimmune, inflammatory, degenerative, viral and prion etiologies have been suggested. Also, recent work suggests myonuclear degeneration and autophagy. Degenerating myonuclei may be indicated by the presence of nuclear and lysosomal proteins in rimmed vacuoles. The loss of myonuclear TDP-43 and the presence of myonuclear protein aggregates further corroborates the evidence of myonuclear involvement in the pathogenesis of IBM.[5] Additionally, the accumulation of autophagy-associated proteins such as p62, LC3, and neighbor of BRCA 1 gene 1 (NBR1) are increased in IBM. [6]
Pathophysiology
Muscle biopsies demonstrate endomysia inflammatory infiltrates predominantly composed of CD8+ T cells and macrophages invading non-necrotic muscle fibers, increased MHC-1 expression on the sarcolemma, and fibers with rimmed vacuoles. Cytochrome oxidase (COX) negative fibers and inclusions on light or electron microscopy are also commonly observed. Amyloid deposits correlating with vacuolated fibers is another characteristic feature that can be demonstrated by the Congo red method. Additionally, there are increased 15 to 18 nanometers cytoplasmic and intranuclear tubulofilamentous inclusions found on electron microscopy when vacuolated fibers are examined. However, rimmed vacuoles may not be seen in as many as 20–30% of muscle biopsies, in such cases, the presence of mitochondrial abnormalities and immunostaining demonstrating TDP-43 and p62 inclusions help to increase diagnostic confidence and distinguish IBM from polymyositis (aside from the clinical pattern of muscle weakness). [7]
Diagnosis
History
Most patients present with slowly progressive limb weakness which might manifest as difficulty getting up from a chair and grip weakness. The predominant sites of weakness and atrophy at onset include the quadriceps, long finger flexors, and ankle extensors. Dysphagia is a common finding however it is rarely the presenting symptoms. Patients have normal life expectancy though they have restricted activities of daily life.[8] [9][10] Facial muscle weakness, particularly orbicularis oculi, is present in many patients and is demonstrated by an inability to close the eyes fully. [11] While orbicularis oculi weakness acquired from IBM is usually not severe enough to cause ocular manifestations, findings such as lagophthalmos, ptosis, and dry eyes are possible with IBM. [12] [13]
Physical examination
The physical examination can provide pertinent information when distinguishing inclusion body myositis other forms of inflammatory myopathies. Features of the physical examination include the following:
- Asymmetric and distal muscle involvement: The finding of more distal, asymmetric weakness with finger and wrist flexor greater than deltoid weakness. And quadriceps weaker than hip flexors is characteristic of IBM.
- Insidious onset: the onset of muscle weakness is gradual. The average duration of symptoms before making a diagnosis is around 5 years.
- Muscle atrophy: weakness in the hands or grip strength due to wasting of finger flexors, wrist flexors. The quadriceps also become weak, which worsens with the progression of the disease.
- Dysphagia: is seen in approximately 30% to 50% of the patients with IBM. It can result in choking, weight loss, aspiration, and pneumonia. Oropharyngeal muscle weakness can result in dysphonia.[9][14]
To detect the subtle weakness of distal finger flexion (typically the earliest exam finding), the clinician should isolate and specifically examine flexion at the distal interphalangeal (DIP) joint. Other muscles that are commonly affected are orbicularis oculi (leading to weakness with eye closure), triceps (weakness with arm extension), and tibialis anterior (leading to foot drop)[15]
Laboratory test
IBM has been linked to the gene variants HLA-DRB1*03:01 and HLA-B*08:01 (components of the 8.1 ancestral MHC haplotype), the CCR5 gene, and TOMM40.[16] Normal or slight elevations of plasma muscle enzyme levels (serum CK less than 10-fold higher than normal) are common with IBM. Autoantibodies against cN1A are common in and highly specific to IBM among muscle diseases and may help distinguish IBM from other forms of myositis. A study diagnostic performance of immunoglobulin M, IgA, and IgG anti-cN1A serum antibodies detected by enzyme-linked immunosorbent assay (ELISA) of all three autoantibody levels resulted in diagnostic sensitivity of 76%.[17] The role of cN-1A antibodies in IBM pathogenesis is unknown but recent research shows that their presence is associated with more severe dysphagia.[18]. Although the reported specificity of cN-1A antibodies is high, cN-1A antibodies are detected in non-IBM patients with various autoimmune disorders: Sjogren’s syndrome (23-36%), systemic lupus erythematosus (14-20%), and dermatomyositis (15%). [19]
Diagnostic procedures
Electromyography and nerve conduction studies may be useful tests to accurately characterize myogenic motor unit involvement in some cases of inclusion body myosis and can help rule out pure motor neurogenic disorders. “Mixed” EMG pattern with myopathic and neuropathic-appearing motor unit potentials is very typical of IBM.[20] However, electromyographic patterns cannot reliably distinguish IBM from other inflammatory myopathies. [21][22] The use of MRI could be helpful due to some disease-specific patterns of muscle involvement in IBM and may help distinguish IBM from polymyositis. Particularly patients who lack classical IBM pathology or those in the early stages of the disease.[23] A muscle biopsy should be performed in most cases of suspected IBM. MRI and EMG may be useful in guiding the selection of a muscle for biopsy, as the diagnostic yield for a biopsy is low in muscles with extensive atrophy or fatty replacement. In some cases, a muscle biopsy is not specific and accurate diagnosis will depend on a combination of clinical findings and a biopsy. Please refer to the pathophysiology section above, briefly, common findings on biopsy include:
- Endomysia inflammatory infiltrate
- Rimmed Vacuoles
- Abundant “Cytochrome Oxidase-Negative” Fibers
- Amyloid deposits in vacuolated fibers identified by Congo red staining.
- Upregulation of MHC Class I and II Expression [24]
- Immunostaining for p62 and TDP-43 [25]
Diagnosis Criteria
The combination of selective weakness of finger flexor, knee extensor, and quadriceps, pathological findings such as endomysial inflammation, and rimmed vacuoles serves as the basis of diagnostic criteria for IBM. There are twenty-four previously proposed IBM diagnostic categories. Twelve of these categories performed with high specificity (≥97%) but varied in their sensitivities (11%–84%). The best performing category was the “European Neuromuscular Centre 2013 probable” with a sensitivity of 84%. [26]
Differential diagnosis
Possible alternative causes of myopathy that resemble inclusion body myositis include:
- Arthritis
- Polymyositis
- Amyotrophic lateral sclerosis [27]
- Hereditary Inclusion-Body Myopathies [28][29]
Management
The current standard of treatment options address the inflammatory and atrophic features of this condition. Trials of interferon beta-1a and methotrexate (MTX) provided moderate-quality evidence of not affecting the progression of IBM. In clinical trials, some therapies have shown to be promising such as anti-T lymphocyte immunoglobulin combined with methotrexate, based on the percentage change in quantitative muscle strength score at 12 months. However, overall IBM has shown to be refractory to these treatments, thus providing evidence that current therapies may not be targeting the affected memory and effector T cells.[30] Another promising therapy, Bimagrumab, an antibody against type II activin receptors, demonstrated an increase in thigh muscle volume at the end of 8 weeks, however in a small clinical trial, did not improve 6-minute walking distance assessed at 52 weeks. Physical therapy and rehabilitation, which include aerobic and low resistance exercises, are critical aspects of treatment. Individually adapted exercise and can maintain function and achieve clinically meaningful improvements in the most affected muscle groups. [31][32][33]
References
- ↑ Mammen AL, Truong A, Christopher-Stine L. Chapter 27. Dermatomyositis, Polymyositis, & Immune-Mediated Necrotizing Myopathy. In: Imboden JB, Hellmann DB, Stone JH, eds. CURRENT Diagnosis & Treatment: Rheumatology, 3e. The McGraw-Hill Companies; 2013. http://accessmedicine.mhmedical.com/content.aspx?aid=57272818
- ↑ Mammen AL. Which nonautoimmune myopathies are most frequently misdiagnosed as myositis? Current Opinion in Rheumatology. 2017;29(6):618-622. doi:10.1097/BOR.0000000000000441
- ↑ 30. Greenberg, S.A. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 15, 257–272 (2019). https://doi.org/10.1038/s41584-019-0186-x
- ↑ Needham M, Corbett A, Day T, Christiansen F, Fabian V, Mastaglia FL. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. Journal of Clinical Neuroscience. 2008;15(12):1350-1353. doi:10.1016/j.jocn.2008.01.011
- ↑ Salajegheh M, Pinkus JL, Taylor JP, et al. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle and Nerve. 2009;40(1):19-31. doi:10.1002/mus.21386
- ↑ D’Agostino C, Nogalska A, Cacciottolo M, King Engel W, Askanas V. Abnormalities of NBR1, a novel autophagy-associated protein, in muscle fibers of sporadic inclusion-body myositis. Acta Neuropathologica. 2011;122(5):627-636. doi:10.1007/s00401-011-0874-3
- ↑ Greenberg SA, Amato AA. Inflammatory Myopathies. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill Education; 2018. http://accessmedicine.mhmedical.com/content.aspx?aid=1175162118
- ↑ Felice KJ, North WA. Inclusion body myositis in Connecticut: Observations in 35 patients during an 8-year period. Medicine. 2001;80(5):320-327. doi:10.1097/00005792-200109000-00006
- ↑ Jump up to: 9.0 9.1 Dimachkie MM, Barohn RJ. Inclusion body myositis. Neurologic Clinics. 2014;32(3):629-646. doi:10.1016/j.ncl.2014.04.001
- ↑ Cox FM, Titulaer MJ, Sont JK, Wintzen AR, Verschuuren JJGM, Badrising UA. A 12-year follow-up in sporadic inclusion body myositis: An end stage with major disabilities. Brain. 2011;134(11):3167-3175. doi:10.1093/brain/awr217
- ↑ Milone M. Diagnosis and Management of Immune-Mediated Myopathies. Mayo Clinic Proceedings. 2017;92:826-837. doi:10.1016/j.mayocp.2016.12.025
- ↑ Badrising UA, Maat-Schieman M, van Duinen SG, et al. Epidemiology of inclusion body myositis in the Netherlands: A nationwide study. Neurology. 2000;55(9):1385-1387. doi:10.1212/wnl.55.9.1385
- ↑ Shams F, Cauchi P. Lagophthalmos and Ptosis in Inclusion Body Myositis. Ophthalmic Plastic & Reconstructive Surgery. 2017;33(3S):S161-S162. doi:10.1097/IOP.0000000000000629
- ↑ Misterska-Skóra M, Sebastian A, Dzięgiel P, Sebastian M, Wiland P. Inclusion body myositis associated with Sjögren’s syndrome. Rheumatology International. 2013;33(12):3083-3086. doi:10.1007/s00296-012-2556-4
- ↑ Engel WK, Askanas V. Inclusion-body myositis: Clinical, diagnostic, and pathologic aspects. In: Neurology. Vol 66. Lippincott Williams and Wilkins; 2006. doi:10.1212/01.wnl.0000192260.33106.bb
- ↑ 30. Greenberg, S.A. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 15, 257–272 (2019). https://doi.org/10.1038/s41584-019-0186-x
- ↑ Greenberg SA. Cytoplasmic 5′-nucleotidase autoantibodies in inclusion body myositis: Isotypes and diagnostic utility. Muscle and Nerve. 2014;50(4):488-492. doi:10.1002/mus.24199
- ↑ 31. Lucchini M, Maggi L, Pegoraro E, Filosto M, Rodolico C, Antonini G, Garibaldi M, Valentino ML, Siciliano G, Tasca G, De Arcangelis V, De Fino C, Mirabella M. Anti-cN1A Antibodies Are Associated with More Severe Dysphagia in Sporadic Inclusion Body Myositis. Cells. 2021; 10(5):1146. https://doi.org/10.3390/cells10051146
- ↑ Herbert MK, Stammen-Vogelzangs J, Verbeek MM, et al. Disease specificity of autoantibodies to cytosolic 5’-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Annals of the Rheumatic Diseases. 2016;75(4):696-701. doi:10.1136/annrheumdis-2014-206691
- ↑ Mastaglia FL, Needham M. Inclusion body myositis: A review of clinical and genetic aspects, diagnostic criteria and therapeutic approaches. Journal of Clinical Neuroscience. 2015;22(1):6-13. doi:10.1016/j.jocn.2014.09.012
- ↑ Lotz BP, Engel AG, Nishino H, Stevens JC, Litchy WJ. Inclusion body myositis: Observations IN 40 patients. Brain. 1989;112(3):727-747. doi:10.1093/brain/112.3.727
- ↑ Tasca G, Monforte M, de Fino C, Kley RA, Ricci E, Mirabella M. Magnetic resonance imaging pattern recognition in sporadic inclusion-body myositis. Muscle and Nerve. 2015;52(6):956-962. doi:10.1002/mus.24661
- ↑ Dion E, Cherin P, Payan C, et al. Magnetic resonance imaging criteria for distinguishing between inclusion body myositis and polymyositis. The Journal of Rheumatology. 2002;29(9).
- ↑ Das L, Blumbergs PC, Manavis J, Limaye VS. Major histocompatibility complex class i and ii expression in idiopathic inflammatory myopathy. Applied Immunohistochemistry and Molecular Morphology. 2013;21(6):539-542. doi:10.1097/PAI.0b013e31827d7f16
- ↑ Dubourg O, Wanschitz J, Maisonobe T, et al. Diagnostic value of markers of muscle degeneration in sporadic inclusion body myositis. Acta Myologica. 2011;30(OCTOBER):103-108. Accessed February 12, 2021. /pmc/articles/PMC3235833/
- ↑ Lloyd TE, Mammen AL, Amato AA, Weiss MD, Needham M, Greenberg SA. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014;83(5):426-433. doi:10.1212/WNL.0000000000000642
- ↑ Solorzano GE, Phillips LH. Inclusion Body Myositis: Diagnosis, Pathogenesis, and Treatment Options. Rheumatic Disease Clinics of North America. 2011;37(2):173-183. doi:10.1016/j.rdc.2011.01.003
- ↑ Ranque-Francois B, Maisonobe T, Dion E, et al. Familial inflammatory inclusion body myositis. Annals of the Rheumatic Diseases. 2005;64(4):634-637. doi:10.1136/ard.2004.025494
- ↑ Rose MR, Jones K, Leong K, et al. Treatment for inclusion body myositis. Cochrane Database of Systematic Reviews. 2015;2015(6). doi:10.1002/14651858.CD001555.pub5
- ↑ 30. Greenberg, S.A. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 15, 257–272 (2019). https://doi.org/10.1038/s41584-019-0186-x
- ↑ Amato AA, Sivakumar K, Goyal N, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83(24):2239-2246. doi:10.1212/wnl.0000000000001070
- ↑ Hanna MG, Badrising UA, Benveniste O, et al. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial. The Lancet Neurology. 2019;18(9):834-844. doi:10.1016/S1474-4422(19)30200-5
- ↑ Alexanderson H. Exercise in Myositis. Current Treatment Options in Rheumatology. 2018;4(4):289-298. doi:10.1007/s40674-018-0113-3

