Ocular Surface Disease in Patients with Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Ocular surface disease
Ocular surface disease represents a spectrum of disorders that affect the surface of the eyes. Dry eye syndrome is one of the most common ocular surface diseases, with incidence ranging from 5.7% to 21.6%.[1][2][3][4] Symptoms of dry eye and ocular surface disease include sensation of dryness, redness, tearing, irritation, burning, foreign body sensation, light sensitivity and intermittent blurred vision. Questionnaires and surveys such as the Ocular Surface Disease Index (OSDI) and Symptom Assessment iN Dry Eye (SANDE) represent methods of characterizing associated symptoms in a systematic manner.[5][6]
Glaucoma
With a prevalence of 3.54%, glaucoma is one of the leading causes of blindness worldwide.[7][8] Symptoms from primary open-angle glaucoma (POAG) generally only arrive when the disease is well advanced portending the importance of screening eye exams and diagnosis in early phases of the disease. The typical signs are related to secondary effects of the optic neuropathy on anatomy of the eye and functional performance with visual field testing. However, given that the most common first line of therapy of glaucoma is topical therapy, development of symptoms from the treatment itself is possibly more prevalent and described below.
Ocular surface disease in patients with glaucoma
Both ocular surface disease and glaucoma are more common in older patients, and first-line therapy for these disease entities are topical medications.[9] They both have a spectrum of disease and severity but have compounding effects when coexistent. This interaction is further complicated by treatments for each condition which can interact and yield counterproductive effects (Figure 1).
Glaucoma treatment may cause chronic inflammation or aggravate a concomitant ocular surface disease. Clinical trials of drugs most often test only one specific medication and preservative at a time, and often only for a short period of time. They do not test multiple or all classes of medications simultaneously for a prolonged period of time. However, in clinical practice patients with glaucoma are typically treated for many years or even decades. Patients may also have preexisting ocular surface disease, dry eye, meibomian gland dysfunction, or chronic allergy and are very frequently treated with more than one topical glaucoma medication. Some ocular surface issues can wax and wane while overall possibly worsening as we get older. It is for these reasons that clinical practice does not always fit into the safety profile of typical clinical trials for medications. There is still no standardized definition nor classification or assessment of glaucoma therapy related ocular surface disease. Hollo et al. defined glaucoma therapy-related ocular surface disease well as, “imbalance of the ocular surface homeostasis caused by the toxic effect of chronic topical medication, which leads to tear film instability, epithelial damage, and inflammation.”[10] A recent study found a number of negative ocular surface markers associated with glaucomatous disease and treatment.[11]
This article will review the manifestations of ocular surface disease in glaucoma patients and review medical and surgical treatments currently available and potential algorithm of management.
Disease Entity
ICD-9
- 370.21 Superficial punctate keratitis
- 375.15 Dry eye syndrome; Tear film insufficiency
- 694.6 Cicatricial pemphigoid
ICD-10
- H04.121 Dry eye syndrome, right
- H04.122 Dry eye syndrome, left
- H04.123 Dry eye syndrome, bilateral
- H16.141 Superficial punctate keratitis, right
- H16.142 Superficial punctate keratitis, left
- H16.143 Superficial punctate keratitis, bilateral
- L12.1 Ocular cicatricial pemphigoid
Manifestations of ocular surface disease in patients with glaucoma
Ocular surface disease in patients with glaucoma can take several manifestations including superficial punctate keratitis, tear-film instability, allergy, pseudopemphigoid and dry eye.
Superficial punctate keratitis
Superficial punctate keratitis (SPK) is seen clinically by using various stains on the cornea and conjunctival surfaces and grading their severity and location. Several authors have demonstrated effects of the medication and preservatives leading to SPK. Many studies have found a significant difference in SPK when comparing treatment with glaucoma medication alone versus with a preservative. The rate of SPK in patients with glaucoma receiving topical therapy can be as high as 18-54%.[12][13][14][15][16][17]
Tear-film instability
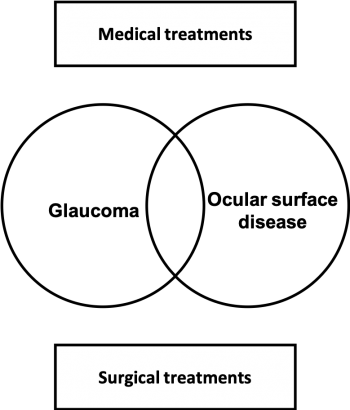
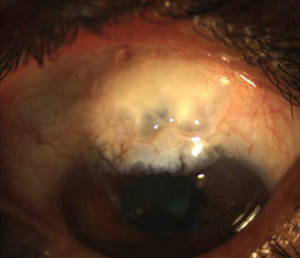
The tear-film instability is functionally measured by tear break up time (TBUT), Schirmer test, osmolarity, and Meibomian gland scores. Studies have reported abnormal TBUT and Schirmer values in over 60% of glaucoma patients.[18][19][20][21][22] Hyperosmolarity is also an important factor but is not completely understood. Figure 2 shows SPK and irregular tear film.
Allergy
Allergic manifestations occur from glaucoma medications as well as their preservatives. They manifest in the form of conjunctival and periorbital hyperemia, chemosis, lid edema and even eczema and itching. The symptoms are hypersensitivity reactions in the form of type 1 to 4. Fortunately, the vast majority of the symptoms resolve after withdrawal of the offending agent. Allergic conjunctivitis and dermatitis to prostaglandin analogs are relatively rare reported at 1.5%.[23] Topical carbonic anhydrase inhibitors can cause dermatitis and conjunctivitis at a rate of 3 to 4%.[24] Beta-blockers have been shown to cause contact dermatitis in 11-13% of patients.[25] Topical alpha-agonist brimonidine can cause a conjunctivitis with follicles in up to 11.5%.[26][27]
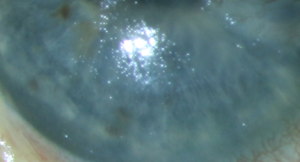
Pseudopemphigoid
Pseudopemphigoid (Figure 3) is a cicatrizing conjunctivitis that mimics ocular mucous membrane pemphigoid in its presentation and requires a conjunctival biopsy to distinguish them. The largest publication to evaluate this disease was by Thorne et al. who showed that 28.3% of all cases of pseudopemphigoid were due to topical glaucoma medications (beta-blockers 87.8%, epinephrine and alpha-agonists 61%, miotics 53.6%).[28] Most of these cases were receiving multiple agents; hence, it was not possible to conclude which medications contributed to the presumed drug-induced pseudopemphigoid.
Dry eye
Common dry eye symptoms include itching, burning, stinging, pain, soreness, sticky eyes, photosensitivity, foreign body sensation, blurred vision, poor vision, redness, intolerance to windy conditions and contact lenses. Despite the importance of symptoms in dry eye, there are currently no gold standard tests to classify them.
Vortex keratopathy
About 20% of patients using netasurdil will show signs of whorl-like subepithelial changes after 4 weeks of use. These, however, do not cause visual compromise and most resolve with discontinuation of the medication.
Evaluation of ocular surface disease
It is not uncommon for patients to have moderate or even severe symptoms in the absence of abnormal ocular surface signs or for patients with severe clinical signs to have relatively mild symptoms. Subjective symptoms typically are assessed by the Ocular Surface Disease Index (OSDI) questionnaire. Objective clinical tests typically include :
- Corneal sensation test using cotton tip applicator or Cochet-Bonnet esthesiometer
- Tear break up time (TBUT)
- Ocular surface staining
- Schirmer test
- Delayed tear clearance
- Meniscometry
- InflammaDry®
- Tear osmolarity
- Tear film interferometry
Impact of ocular surface disease on quality of life
Both ocular surface disease and glaucoma can have affect the quality of life. There are several validated quality of life questionnaires that correlate closely with clinical indices of severity. Poorer quality of life scores are associated with worse functional status and increased visual morbidity. Several studies have evaluated the quality of life in glaucoma patients with ocular surface disease. Camp et al. found an increasing number of glaucoma drops was significantly associated with an increased percentage of severe dry eye symptoms.[29] There was also an association between increasing number of drops and decreasing emotional well-being scores based on Impact of Dry Eye on Everyday Life questionnaire (Alcon, Fort Worth, TX). Skalicky et al. found that ocular surface disease is more common with increasing glaucoma severity and is associated with poorer visual function and glaucoma-related quality of life and also higher exposure to benzalkonium chloride (BAK).[30]
Preservatives versus alternatives
Preservatives have been used for multiple-use containers for many years. The most widely used is BAK, a quaternary ammonium compound with bacteriostatic, bactericidal, and surfactant properties.[31][32] BAK destroys cell membrane but as Baudoin stated, “it is almost possible for a chemical to discriminate membranes of pathogens from those of normal eye cell.” [33] More recently, a few preservative alternatives have been developed, such as SofZia, which is commonly found in travoprost, or Purite, an oxidative preservative commonly found in formulations of brimonidine. There are also several preservative-free formulations for single use available, giving more options for patients. A few examples include timolol maleate, fixed combination of dorzolamide and timolol, as well as tafluprost. Table 1 summarizes glaucoma medications and dry eye medications that have non-BAK preservatives.

Preservatives and their impact on ocular surface
There is an extensive body of literature describing the harmful effects of preservatives, especially BAK in both in-vitro and animal studies. However, preparations containing BAK continue to be found in generic formulations of glaucoma medications. One reason for this is the harmful effects of BAK are time and dose dependent such that in small enough quantities the harmful effects are often well tolerated. However, when patients require multiple medications taken multiples times per day, the negative effects add up and lead to more severe reactions on the ocular surface. Human and animal cell cultures have been used to study the effects of mainly BAK.[33][34][35] Often times these studies evaluate cell death as outcomes. The concentration of BAK and exposure time to BAK vary greatly, which make the clinical applicability limited.
Two very large studies evaluated signs and symptoms of ocular surface disease in glaucoma patients with preservative-containing medications and preservative-free medications, and both found higher rates of ocular surface signs and symptoms in the preservative-containing groups.[14][17] They also found that after switching to preservative-free formulations, signs and symptoms significantly improved. In another study, the severity of ocular surface disease symptoms was correlated with the number of glaucoma medications used.[36] In a prospective study by Ishibashi et al., BAK preserved timolol was shown to have negative effects on the corneal tear-film stability and epithelial barrier function.[37] TBUT has been shown to be affected by BAK in normal healthy eyes as well.[38] In another study, Bron et al. switched 435 patients treated with preserved timolol to preservative free timolol, and after 3 months, pressure control was maintained while dry eye, foreign body sensations were reduced by half. The percentage of conjunctival hyperemia reduced from 24.4% to 14.6%. Superficial punctate keratitis rates were also reduced by half.[39] Recently, Uusalito et al. reported switching 339 patients from BAK-containing latanoprost to preservative-free tafluprost which resulted in significantly decreased signs and symptoms of ocular surface disease.[40]
Purite® (oxychloro complex)
Noecker et al. studied the effects of Purite, an oxidative preservative in rabbit eyes.[41] In this study, patients treated with glaucoma medications containing BAK resulted in greater damage to the cornea and conjunctiva compared with lower concentrations of BAK. Purite oxidizes microbial cellular components but has no significant effect on human ocular tissues as opposed to BAK, which has effects on all cells regardless of organism namely bacterial or human. Mundorf et al. demonstrated similar pressure reduction with fewer subjective symptoms from patients when treated with Purite-containing brimonidine versus BAK containing brimonidine.[42] Later Cantor et al. demonstrated similar pressure reduction with 0.1% and 0.15% brimonidine-purite, leading to similar effect with less exposure of the ocular surface to the active compound as well as with no BAK.[43]
SofZia®
Another approach to avoiding BAK is a preservative in travoprost called SofZia, an ionic-buffered preservative. SofZia causes oxidative damage and subsequent death in bacteria that lack the enzymes cytochrome oxidase or catalase. Human cells possess these enzymes and are thus not similarly harmed. It has been demonstrated in animal models to cause significantly less corneal epithelial damage as compared to BAK.[44] Henry et al. treated 691 patients with BAK preserved latanoprost or bimatoprost and switched to SofZia preserved travoprost with significant improvement in ocular surface symptoms and hyperemia without a compromise in pressure control.[45]
Preservative-free medications
Another option to BAK is to use medications with no preservative, typically in the form of individual dose packets of medications. While a patient may benefit from less BAK and even no BAK, the cost can be significantly different from generics to brand name medications. This may be a limiting factor for many patients who suffer from both glaucoma and ocular surface disease. By using preservative-free medications, the active ingredient can still lead to ocular surface disease, but this is much less frequent. Secondary microbial contamination of containers for medication is a known byproduct of eliminating preservatives; however, translation into clinical infection is less known.[46]
Management
Medical treatments
Avoiding preservatives
It has been fairly well demonstrated that BAK is either the primary offending agent or compounding signs and symptoms from the active drug of ocular surface disease in glaucoma patients. It has also been fairly well demonstrated that the more BAK used meaning total accumulation of BAK over time or per day can increase the signs and symptoms of ocular surface disease. A stepwise approach of substitution of agents may be warranted for mild cases of ocular surface disease. A complete overhaul of all topical therapies may leave some causative questions unanswered especially in patients on multiple therapies in mildly symptomatic cases. Since the effects are dose response related (i.e., multiple agents with BAK) then stepwise decrease the number of treatments with the same offending agent may provide a controlled decrease in exposure while balancing the desired therapeutic effects. Combination medications can be used to decrease the overall BAK amount. If using several medications all with BAK then switching 1 BAK medication for a non-BAK may be enough. If that is not enough then switching all medications away from BAK is another trial to help improve the ocular surface disease. If the ocular surface is severely debilitated then likely much larger overhaul of therapy may be needed with elimination of as many causative factors simultaneously to control the problem. When changing topical agents, consideration of onset of action and washout period may influence how to pattern the treatment algorithm.
Lubricants without added preservatives
In standard dry eye therapy, artificial tears and lubricants are used. When they are added to a patient’s regimen, the total dose of number of drops per day of preservative especially BAK should be noted. If there are relatively few drops per day and the symptoms are mild using any artificial tear may be helpful. There are artificial tears that do not contain BAK and some are preservative free. Especially in cases of severe ocular surface disease and when a patient needs several drops of lubrication per day, it is advisable to use preservative-free tears.
Topical cyclosporine
Both Restasis® (0.05%) and Cequa® (0.09%) are cyclosporin A solutions that have been used in ocular surface disease.[47] Saini et al. reported improvements in ocular surface disease signs and symptoms when using this medication concurrently with other antiglaucoma medications in chronic glaucoma patients.[48] Preliminary studies suggest Cequa® may be more effective and better tolerated than Restasis®[49][50]
Xiidra® (lifitegrast ophthalmic solution)
In 2016, the FDA approved a new drug called Xiidra (lifitegrast ophthalmic solution) for treatment of dry eye syndrome. This topical agent is a novel T-cell inhibitor, which suppresses the inflammatory processes harmful to the ocular surface.[51] More importantly, Xiidra ophthalmic solution comes in preservative-free, single-dose containers, further protecting the surface of the eyes.
Placement of novel drug delivery systems
Researchers have recently introduced a few novel drug delivery systems that may lower IOP while potentially limit patient's non-compliance, as well as surface toxicity.
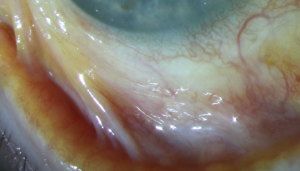
Bimatoprost SR (Allergan, Dublin, Ireland)
DurystaTM is a commercially available biodegradable solid matrix polymer (Figure 4). This device is placed in the anterior chamber(usually in the inferior angle) via intracameral injection through a 28 gauge needle and can deliver bimatoprost to lower IOP for up to 24 months.[52]. This is contraindicated in patients with endothelial disease or with any obstruction of the angle.
Bimatoprost Ring (Allergan, Dublin, Ireland)
Phase 2 and open-label extension (OLE) of this silicone matrix ring has been completed. This insert is placed circumferentially in the fornices using a similar technique to placing a contact lens and can deliver bimatoprost to lower IOP for up to 6 months. Follow-up in OLE showed higher incidence of mucus compared to placebo (21.3% vs 13.6%); however, this side effect reportedly improved dry eye symptoms in patient with history of dry eye.[53]
Piezo-Print Microdose Delivery (Eyenovia, New York, NY, USA)
Phase 3 trial of this microdose dispenser termed OptejetTM for latanoprost is in progress. This device works by delivering a collimated stream of medication droplets faster than the eye's blink reflex (80ms vs 100ms), and thus coats the ocular surface with just enough medication and preservatives that can achieve therapeutic effects while potentially reduce ocular surface symptoms at the same time.[54]
iDose (Glaukos, San Clemente, CA, USA)
This travoprost-loaded titanium implant was FDA approved in 2023. This device is placed into the sclera through the Schlemm's canal, using a similar technique to the iStent® inject and can deliver travoprost into the anterior chamber to lower IOP for up to 12 months before being replaced. Since delivery of travoprost bypasses the ocular surface, harmful effects of the medication and preservatives to the ocular surface can be avoided. However, placement of iDose requires a clear corneal incision, and patients will need annual replacement of this device. [55]
Surgical treatments
Consideration for early selective laser trabeculoplasty (SLT)
One of the strategies to minimize potential harmful topical medications is to not use them at all. Considering SLT earlier in treatment algorithm may be helpful. In a general sense, utilizing SLT can be thought of as reducing the medical burden by 1 drop in many patients.
Punctal occlusion
Punctal occlusion has also been used for the treatment of dry eyes.[56] Punctal occlusion can have different effects in a glaucoma patients as opposed to the standard patients with dry eyes. If the outflow of tears from the ocular surface is slowed, the ocular surface has more liquid. It will retain and absorb more of the active drug but also any potential effect from the preservatives. Optiz et al. demonstrated an improved pressure reduction from punctal occlusion but did not study the effects on the ocular surface.[57] Punctal occlusion in the from of plug or cautery can be utilized in glaucoma patients but a patient’s outcome should be monitored for benefits and also possible side effects.
For the past few years, the researchers are developing and testing a new type of punctal plug. This plug can be embedded with antiglaucoma medications, which are slowly delivered over time. Two preservative-free medication-embedded plugs, namely Latanoprost Punctal Plug Delivery System (L-PPDS, Mati Therapeutics, Austin, TX, USA) and Travoprost Insert (OTX-TP, Ocular Therapeutix, Bedfort, MA, USA), have been taken to clinical trials recently.[58] If these treatment modalities are employed, time will answer whether the surface toxicity from preservatives remains an issue.
Minimally invasive glaucoma surgery (MIGS)
Today we have a wide variety of MIGS.; some are stand alone and some are easily combined with cataract surgery. If a cataract is present, and 1 or more topical therapies are controlling IOP but causing ocular surface issues, then MIGS alone or in combination with cataract surgery may yield benefits not only to the pressure but also to the ocular surface. Figure 5 shows an iStent inject®, the first micro-size implant approved by the FDA for MIGS, bypassing the trabecular meshwork to lower IOP. When the ocular surface disease is improved, it will likely lead to a happier patient with an improved quality of life and also improved compliance.[59]
Benefits versus risks of surgical treatments
Patients with advanced glaucoma often are on multiple medications. These scenarios are the ones that frequently lead to ocular surface disease that may results in worsened compliance and thus worsening glaucomatous damage. The decision to perform incisional surgery is never taken lightly as trabeculectomies and glaucoma drainage devices can be humbling surgeries due to their complication profiles. One benefit from their use is better pressure control which usually leads to lower topical medication use. The decrease in topical glaucoma medication use can lead to a real improvement in the ocular surface in most cases. However, trabeculectomies and glaucoma drainage devices can also lead to new ocular surface problems from exposure to toxic medication such as mitomycin C and 5-fluorouracil and also anatomic problems such as bleb dysesthesia (Figure 6).[60] Aqueous shunting devices are not immune to causing ocular surface pain either.[61]
Conclusion
Ocular surface disease in glaucoma patients occurs at high rates, which increases with age and often with the severity of glaucoma. Fortunately, we have several preservative free topical medications as well as alternate preservatives other than the historically toxic BAK. We also have a variety of laser and surgical options to lower the medication burden, therefore helping the ocular surface disease as well as the glaucoma.
References
- ↑ Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001;45(suppl2):S199–S202.
- ↑ Moss SR, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2001;118:1264–1268.
- ↑ Moss SE, Klein R, Klein BE. Long-term incidence of dry eye in an older population. Optom Vis Sci. 2008;85:668–674.
- ↑ Schaumberg DA, Sullivan DA, Buring JE, et al. Prevalence of dry eye syndrome among us women. AM J Ophthalmol. 2003;136:318–326.
- ↑ Amparo F, Schaumberg DA, Dana R. Comparsison of two questionnaires for dry eye symptom assessment: the ocular surface disease index and the symptom assessment in dry eye. Ophthalmology. 2015;122:532–538.
- ↑ Friedman DS, Wolfs RCW, O’Colmain, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538.
- ↑ Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systemic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090.
- ↑ Varma R, Lee PP, Goldberg I, et al. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515–522.
- ↑ Ramulu PY, Corcoran KJ, Corcoran SL, et al. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114:2265–2270.
- ↑ Hollo G, Katsanos A, Boboridis KG, et al. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: efficacy, safety and potential advantages. Drugs. 2018;78:39–64.
- ↑ Srivastava K, Bhatnagar KR, Shakrawal J, Tandon M, Jaisingh K, Pandey L, Roy F. Ocular surface changes in primary open-angle glaucoma on anti-glaucoma medications versus treatment-naïve patients. Indian J Ophthalmol. 2023 Dec 15. doi: 10.4103/IJO.IJO_618_23. Epub ahead of print. PMID: 38099355.
- ↑ Aihara M, Otani S, Kozaki J, et al. Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J Glaucoma. 2012;21:60–64.
- ↑ Inoue K, Okugawa K, Kato S, et al. Ocular factors relevant to anti-glaucomatous eyedrop-related keratoepitheliopathy. J Glaucoma. 2003;12:480–485.
- ↑ Jump up to: 14.0 14.1 Jaenen N, Baudouin C, Pouliquen P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17:341–349.
- ↑ Johnson DH, Kenyon KR, Epstein DL, et al. Corneal changes during pilocarpine gel therapy. Am J Ophthalmol. 1986;101:13–15.
- ↑ Maas S, Ros FE, De Heer LJ, et al. Long-term treatment with Pilogel/beta-blocker in glaucoma patients. Int Ophthalmol. 1991;15:281–284.
- ↑ Jump up to: 17.0 17.1 Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–423.
- ↑ No author listed. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92.
- ↑ Arita R, Itoh K, Maeda S, et al. Effects of long-term topical anti-glaucoma medications on meibomian glands. Graefes Arch Clin Exp Ophthalmol. 2012;250:1181–1185.
- ↑ Arita R, Itoh K, Maeda S, et al. Comparison of the long-term effects of various topical antiglaucoma medications on meibomian glands. Cornea. 2012;31:1229–1234.
- ↑ Januleviciene I, Derkac I, Grybauskiene L, et al. Effects of preservative-free tafluprost on tear film osmolarity, tolerability, and intraocular pressure in previously treated patients with open-angle glaucoma. Clin Ophthalmol. 2012;6:103–109.
- ↑ Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355.
- ↑ Baudouin C. Allergic reaction to topical eyedrops. Curr Opin Allergy Clin Immunol. 2005;5:459–463.
- ↑ Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28:267–282.
- ↑ Jappe U, Uter W, Menezes de Padua CA, et al. Allergic contact dermatitis due to beta- blockers in eye drops: a retrospective analysis of multicentre surveillance data 1993-2004. Acta Derm Venereol. 2006;86:509–514.
- ↑ Katz LJ. Brimonidine tartrate 0.2% twice daily vs timolol 0.5% twice daily: 1-year results in glaucoma patients. Brimonidine Study Group. Am J Ophthalmol. 1999;127: 20–26.
- ↑ Schuman JS, Horwitz B, Choplin NT, et al. A 1-year study of brimonidine twice daily in glaucoma and ocular hypertension. A controlled, randomized, multicenter clinical trial. Chronic Brimonidine Study Group. Arch Ophthalmol. 1997;115:847–852.
- ↑ Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopem- phigoid. Ophthalmology. 2004;111:45–52.
- ↑ Camp A, Wellik SR, Tzu JH, et al. Dry eye specific quality of life in veterans using glaucoma drops. Cont Lens Anterior Eye. 2015;38:220–225.
- ↑ Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153:1–9; e2.
- ↑ Caraccio TR, McGuigan MA. Benzalkonium chloride. In: RC Dart, ed. Medical toxicology, 3rd ed. New York, NY: Lippincott Williams & Wilkins; 2004:1255–1257.
- ↑ Yee RW. The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr Opin Ophthalmol. 2007;18:134–139.
- ↑ Jump up to: 33.0 33.1 Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86:716–726.
- ↑ Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334.
- ↑ Tressler CS, Beatty R, Lemp MA. Preservative use in topical glaucoma medications. Ocul Surf. 2011;9:140–158.
- ↑ Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–621.
- ↑ Ishibashi T, Yokoi N, Kinoshita S. Comparison of the short-term effects on the human corneal surface of topical timolol maleate with and without benzalkonium chloride. J Glaucoma. 2003;12:486–490.
- ↑ Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998;82: 39–42.
- ↑ Bron A, Chiambaretta F, Pouliquen P, et al. Efficacy and safety of substituting a twice- daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertension. J Fr Ophtalmol. 2003;26: 668–674.
- ↑ Uusitalo H, Egorov E, Kaarniranta K, et al. Benefits of switching from latanoprost to preservative-free tafluprost eye drops: a meta-analysis of two Phase IIIb clinical trials. Clin Ophthalmol. 2016;10:445–454.
- ↑ Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–496.
- ↑ Mundorf T, Wilcox KA, Ousler GW III, et al. Evaluation of the comfort of Alphagan P compared with Alphagan in irritated eyes. Adv Ther. 2003;20:329–336.
- ↑ Cantor LB, Safyan E, Liu CC, et al. Brimonidine-purite 0.1% versus brimonidine- purite 0.15% twice daily in glaucoma or ocular hypertension: a 12-month randomized trial. Curr Med Res Opin. 2008;24:2035–2043.
- ↑ Kahook MY, Noecker RJ. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with sofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tears. Cornea. 2008;27:339–343.
- ↑ Henry JC, Peace JH, Stewart JA, et al. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. Clin Ophthalmol. 2008;2:613–621.
- ↑ Rahman MQ, Tejwani D, Wilson JA, et al. Microbial contamination of preservative free eye drops in multiple application containers. Br J Ophthalmol. 2006;90:139–141.
- ↑ Kymionis GD, Bouzoukis DI, Diakonis VF, et al. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008;2:829–836.
- ↑ Saini M, Dhiman R, Dada T, et al. Topical cyclosporine to control ocular surface disease in patients with chronic glaucoma after long-term usage of topical ocular hypotensive medications. Eye (Lond). 2015;29:808–814.
- ↑ Luchs J. Phase 3 clinical results of cyclosporine 0.09% in a new nanomicellar ophthalmic solution to treatment keratoconjunctivitis sicca. American society of cataract and refractive surgery (ASCRS) annual meeting; 2018; Washington, DC.
- ↑ Tauber J, Schechter BA, Bacharach J, et al. A phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–1929. doi:10.2147/OPTH.S175065
- ↑ Afroz Abidi, Pooja Shukla, Ali Ahmad. Lifitegrast: a novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194–198.
- ↑ Lewis, R.A., Christie, W.C., Day, D.G., et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am. J. Ophthalmol. 2017;175:137–147.
- ↑ Brandt JR, DuBiner HB, Benza R, Sall KN, Walker GA, Semba CP. Long-term Safety and Efficacy of a Sustained-Release Bimatoprost Ocular Ring. Ophthalmology. 2017; 124(10):1565—1566.
- ↑ Pasquale LR, Lin S, Weinreb RN, Tsai JC, Kramm RL, Ianchulev T. Latanoprost with high precision, piezo-print microdose delivery for IOP lowering: clinical results of the PG21 study of 0.4 µg daily microdose. Clin Ophthalmol. 2018; 12: 2451—2457.
- ↑ Baker-Schena L. Novel drug delivery system. EyeNet Magazine. 2019;4:47–52.
- ↑ Ervin AM, Law A, Pucker AD. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 2017;6:CD006775.
- ↑ Opitz DL, Tung S, Jang US, et al. Silicone punctal plugs as an adjunctive therapy for open-angle glaucoma and ocular hypertension. Clin Exp Optom. 2011;94:438–442.
- ↑ Jump up to: 58.0 58.1 Barar J, Aghanejad A, Fathi M, Omidi Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts. 2016;6(1):49-67.
- ↑ De Keyser M, De Belder M, De Groot V. Quality of life in glaucoma patients after selective laser trabeculoplasty. Int J Ophthalmol. 2017;10:742–748.
- ↑ Masoumpour M, Nowroozzadeh MH, Razeghinejad MR. Current and future techniques in wound healing modulation after glaucoma filtering surgeries. The Open Ophthalmol Journal. 2015; 10:68–85
- ↑ De Keyser M, De Belder M, De Groot V. Quality of life in glaucoma patients after selective laser trabeculoplasty. Int J Ophthalmol. 2017;10:742–748.