Meibomian Gland Dysfunction (MGD)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Meibomian Gland Dysfunction (MGD) is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
- ICD-10:H02.89
- Short Description: Other specified disorders of eyelid
Disease
Meibomian glands or glandulae tarsales are large sebaceous glands present in eyelids which secrete lipids that form the superficial layer of tear film to protect evaporation of the aqueous phase. These glands were first described in detail by German physician Heinrich Meibom (1638-1700) and named after him (Fig 1).[1] There are about 25-40 glands in the human upper eyelid and about 20-30 in the lower eyelid with lengths of about 5.5 mm in upper eyelid versus 2 mm in lower eyelid in Caucasian eyes.[2] [3]Each gland consists of clusters of about 10-15 secretory acini in the upper eyelid which secrete lipid material through small ductules via a central duct[4] at the lid margin contributing to the outermost layer of the tear film. The contraction of the orbicularis muscle during blinking and the contraction of Riolan muscle at the terminal part of the ductal system generates mechanical force that facilitates the secretion of meibum.[5] Meibum contains over 100 major individual complex mixture of lipids, over 90 proteins, electrolytes that contribute to the stability of tear film in health and disease.[6] [7]Aging, diet, sex hormones, systemic and ocualar surface inflammatory disorders, usage of antibiotics and primary dysfunction of Meibomian glands alters the composition of lipids and proteins in meibum resulting in altered tear film stability and function.
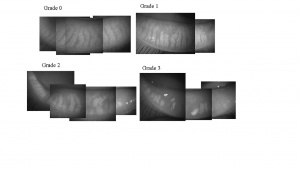
Korb and Henriquez coined the term Meibomian Gland Dysfunction (MGD) in a study addressing contact lens intolerance and the obstruction of the Meibomian gland orifices by desquamated epithelial cells. [8] The International Workshop on MGD defined the disease as “a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/ quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease”.[9] Images of normal and progressive gland loss in patients with MGD are shown in Figure 2.[10]
Epidemiology
MGD is an underdiagnosed and undertreated disease with asymptomatic disease being far more common than the symptomatic MGD.[11][12]It is estimated that 70% of Americans over the age of 60 have MGD.[13] The prevalence is less among Caucasians when compared to Asians and it ranges from 3.5-70% depending on the parameter looked at.[14][15]For example, the prevalence ranges from 61-69.3% based on telaniectasia among Asians while it ranges from 3.5-19.9% among Caucasians. Furthermore, the prevalence increases with age and appears to be higher in males when compared to females.
Risk Factors
Risk factors of MGD include aging, deficiency of sex hormones notably androgens, other systemic conditions such as Sjogren’s syndrome (SS), Stevens-Johnson Syndrome (SJS), psoriasis, atopy, polycystic ovary syndrome (PCOS) and hypertension.[4][10][16]In addition, ophthalmic factors namely aniridia, chronic blepharitis, contact lens wear, eyelid tattooing, trachoma and Demodex folliculorum infestation have been shown to impact Meibomian gland function. Use of antibiotics, Isotretinoin for Acne, antihistamines, antidepressants, and hormone replacement therapy are found to be associated with MGD.
Classification
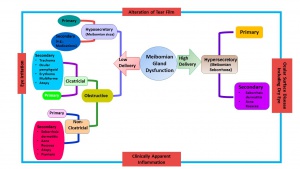
The new classification system proposed by the 2011 International Workshop on MGD is shown in Figure 3. [9] MGD is classified based on the final consequence of Dry Eye Diseases (DED) into low delivery and high delivery categories based on the secretion status. Low delivery status is further classified into hyposecretory and obstructive conditions. Hyposecretion is due to decreased Meibomian gland function from gland atrophy or medications. The obstruction of meibomian glands is the most common cause for hyposecretion which results from the hypertrophy of ductal epithelium and keratinization due to aging, or from decreased expression of androgen receptors, or medications. Obstructive MGD can be further classified into cicatricial and noncicatricial. Hypersecretory MGD results from excessive secretion of lipids. MGD leads to alterations in tear film, eye irritation, ocular surface disease including dry eye and clinically apparent inflammation.
Pathophysiology
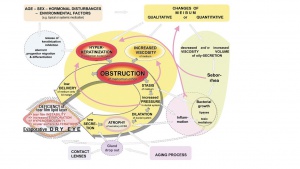
MGD is a highly complex disease condition that is associated with or caused by several host, microbial, hormonal, metabolic and environmental factors. The pathways involved in the pathophysiology of MGD proposed by the 2011 International Workshop on Meibomian Gland Dysfunction are shown in Figure 4.[4][17]A comprehensive double vicious circle which takes DED and MGD pathophysiologies into consideration has been proposed recently.[17] As depicted in the Figure 4, obstruction due to increased viscosity of meibum or hyperkeratinization Meibomian gland ductal system leads to decreased secretion of meibum affecting the tear film’s stability leading to dry eyes. A number factors such as aging process, alterations in sex hormones and the expression of their receptors, nutritional status, reduced blinking of eyes, medications and infections and disease conditions such as seborrheic dermatitis influence physical and functional status of Meibomian glands leading to loss of function, morphologic changes, atrophy and gland drop out.[16][13][18][19]Significant alterations in the expression of 400 genes have been reported in meibomian glands in MGD.[20] Furthermore, these changes also impact the composition of meibum leading to instability of tear film.[21][22][23][24]Staph aureus incidence was higher and Coagulase negative staphylococcus, Corynebacterium and streptococci incidence was lower in patients with mild and moderate to severe disease in lid margin swabs after gland expression.[25]
Diagnosis
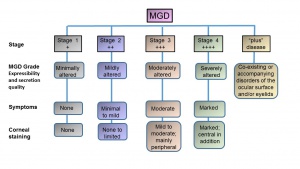
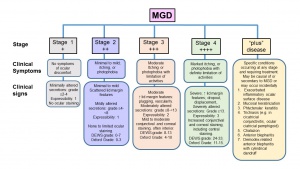
The diagnosis of MGD is based on examining the ocular surface and lid margin tear meniscus in association with altered anatomical features such as terminal duct obstruction, gland drop out, qualitative and quantitative changes in meibum and pathological events leading to MGD. The Diagnostic subcommittee at the International Workshop on Meibomian Gland Dysfunction recommended several diagnostic tests for MGD and proposed two approaches for diagnosing symptomatic MGD-related disease.[26] For asymptomatic adults, performing gland expression with digital pressure to the central lower lid followed by assessing ocular surface damage is recommended. Presence of ocular surface damage or anatomical changes warrants Meibomian gland functionality assessment. Staging of the disease including clinical symptoms and signs are shown in Figures 5 and 6.
- Administer symptoms questionnaire eg. OSDI questionnaire
- Measure blink rate and blink interval:
- Measure lower tear meniscus height
- Measure tear osmolarity
- Ocular surface staining: Assess epithelial cell damage. Oxford Grading System, Dry Eye WorkShop (DEWS) grading
- Break up time
- Tear break up time (TBUT): Normal 15-45 seconds
- Fluorescein break up time (FBUT):Normal range >10 seconds
- Noninvasive break up time (NIBUT): Normal range 40-60 seconds
- Schirmer test:<5 mm/5 min
- If MGD (asymptomatic or symptomatic is not diagnosed earlier)
- Quantify morphologic lid features:
- Expressibility of meibum and its quality
- Meibography: Document morphology infra-red or near infra-red video cameras, confocal microscopy, spectral-domain optical coherence tomography (SD-OCT)[3][10][27][28][29].
- Symptoms assessment {ocular surface disease index (OSDI) and dry eye questionnaire (DEQ)
- Measure of osmolarity
- Tear secretion test
- Measurement of tear volume
- Tear evaporation rate (Evaporimetry)
- Corneal and conjunctival staining
- Tests to assess ocular inflammation
Clinical Management and Treatment
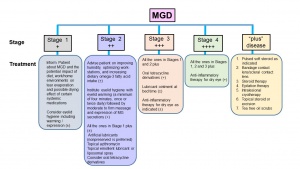
The report of the subcommittee on Management and Treatment of MGD at the International Workshop on MGD recommended Staged Treatment Algorithm as shown in Figures 7.30[30] As shown in Figure 5, MGD is classified into different stages depending on expressibility and secretion quality, severity of symptoms and corneal staining to guide the treatment. The clinical signs and symptoms varies with different stages of the disease (Figure 6) that guide the treatment options (Figure 7).
Topical lubricants are advocated to relieve symptoms, reduce tear film evaporation and stabilize lipids in tear film. However, lid hygiene and warm compress or heat applications are the mainstay of the clinical management. Gentle massage, application of heat to eyelids with warm compress such as hot wet towel or with heat masks, or with devices (LipiFlow Thermal Pulsation System, MiBo Thermaflow, BlephEx, Intense Pulse Tx, KCL1100 etc.) that help liquefaction of meibum and prevent tear evaporation, topical or systemic antibiotics to control infections and treating Demodex mite infestation with tea tree oil (TTO) help restore meibomian gland’s function.[31] [32][33][34][35][36]The treatment algorithm was found to be effective 3 and 6 months post-follow up in a recent study consisting of 108 subjects with DED and MGD.[37]
Medical therapy
Clinical trial: Oral azithromycin versus doxycycline
A 2015 clinical trial of oral azithromycin versus doxycycline was done to assess response in meibomian gland dysfunction (MGD).[38]
Objectives
The goal was to determine the efficacy and safety of oral azithromycin compared to doxycycline in patients with meibomian gland dysfunction (MGD) who had failed to respond to prior conservative management.
Methods
Clinical trial randomly assigning oral 5-day azithromycin (500 mg on day 1 and then 250 mg/day) or 1-month doxycycline (200 mg/day). Patients were >12 years old with posterior blepharitis who had not responded to conservative management: eyelid warming/massage/cleaning (4–5 min) twice a day and artificial tears (four times a day). They also continued eyelid warming/cleaning and artificial tears. A score comprising five symptoms and seven signs (primary outcome) was recorded before treatment and at 1 week, and 1 and 2 months after treatment. A total score was the sum of both scores at each follow-up. Side effects were recorded, and overall clinical improvement was categorized as excellent, good, fair, or poor based on the percentage of change in the total score.
Main outcome measures
Symptoms and signs related to MGD.
Limitations
Absence of a control group without any systemic medication.
Results
110 patients were included to receive either oral azithromycin for 5 days or doxycycline for 1-month. MGD symptoms and signs improved in both groups. The azithromycin group showed a significantly better overall clinical response, with more improvement of the bulbar conjunctival redness, and ocular surface staining. Doxycycline group had significantly more side effects.
Conclusions
Although both oral azithromycin and doxycycline improved the symptoms of MGD, 5-day oral azithromycin is recommended for its better effect on improving the signs, better overall clinical response and shorter duration of treatment.
Pearls for clinical practice
Oral 5-day azithromycin (500 mg on day 1 and then 250 mg/day) is a good option to treat MGD.
Surgical therapy
Surgical therapy is not infrequently indicated when secondary consequences of chronic MGD cause morbidity to the patients. These include multiple and/or recurrent chalaza, meibomian gland inversion, trichiasis and distichiasis and rarely cicatricial entropion with keratopathy. Surgical procedures for the above include incision and curettage, or intralesional triamcinolone for subacute lesions, technical or electroepilation of symptomatic eyelashes and even surgical entropion correction for moderate to severe cicatricial entropion. Recurrence are not uncommon with realistic expectations of the patient is emphasised.
Additional Resources
- Meibomian Glands. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/anatomy/meibomian-glands-list. Accessed March 18, 2019.
References
- ↑ Meibomius H. De vasis palpebrarum novis epistola: Muller, Helmstadt; 1666.
- ↑ Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye (Lond) 1991;5 ( Pt 4):395-411.
- ↑ Jump up to: 3.0 3.1 Shirakawa R, Arita R, Amano S. Meibomian gland morphology in Japanese infants, children, and adults observed using a mobile pen-shaped infrared meibography device. Am J Ophthalmol 2013;155:1099-103 e1.
- ↑ Jump up to: 4.0 4.1 4.2 Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci 2011;52:1938-78.
- ↑ Linton RG, Curnow DH, Riley WJ. The Meibomian Glands: An Investigation into the Secretion and Some Aspects of the Physiology. Br J Ophthalmol 1961;45:718-23.
- ↑ Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol 2006;90:372-7.
- ↑ Shrestha RK, Borchman D, Foulks GN, Yappert MC, Milliner SE. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults, and adults with meibomian gland dysfunction using (1)H-NMR spectroscopy. Invest Ophthalmol Vis Sci 2011;52:7350-8.
- ↑ Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc 1980;51:243-51.
- ↑ Jump up to: 9.0 9.1 Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 2011;52:1930-7.
- ↑ Jump up to: 10.0 10.1 10.2 Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008;115:911-5.
- ↑ Viso E, Rodriguez-Ares MT, Abelenda D, Oubina B, Gude F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci 2012;53:2601-6.
- ↑ Yeotikar NS, Zhu H, Markoulli M, Nichols KK, Naduvilath T, Papas EB. Functional and Morphologic Changes of Meibomian Glands in an Asymptomatic Adult Population. Invest Ophthalmol Vis Sci 2016;57:3996-4007.
- ↑ Jump up to: 13.0 13.1 Chader GJ, Taylor A. Preface: The aging eye: normal changes, age-related diseases, and sight-saving approaches. Invest Ophthalmol Vis Sci 2013;54:ORSF1-4.
- ↑ Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52:1994-2005.
- ↑ Foulks GN, Nichols KK, Bron AJ, Holland EJ, McDonald MB, Nelson JD. Improving Awareness, Identification, and Management of Meibomian Gland Dysfunction. Ophthalmology 2012;119:S1-S12.
- ↑ Jump up to: 16.0 16.1 Mantelli F, Moretti C, Macchi I, et al. Effects of Sex Hormones on Ocular Surface Epithelia: Lessons Learned From Polycystic Ovary Syndrome. J Cell Physiol 2016;231:971-5.
- ↑ Jump up to: 17.0 17.1 Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol 2016;100:300-6.
- ↑ Uchino M, Dogru M, Yagi Y, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci 2006;83:797-802.
- ↑ Souchier M, Joffre C, Gregoire S, et al. Changes in meibomian fatty acids and clinical signs in patients with meibomian gland dysfunction after minocycline treatment. Br J Ophthalmol 2008;92:819-22.
- ↑ Liu S, Richards SM, Lo K, Hatton M, Fay A, Sullivan DA. Changes in Gene Expression in Human Meibomian Gland Dysfunction. Investigative Ophthalmology & Visual Science 2011;52:2727-40.
- ↑ McCulley JP, Shine WE. The lipid layer of tears: dependent on meibomian gland function. Exp Eye Res 2004;78:361-5.
- ↑ Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea 1996;15:340-6.
- ↑ Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea 2004;23:781-3.
- ↑ Joffre C, Souchier M, Gregoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol 2008;92:116-9.
- ↑ Watters GA, Turnbull PR, Swift S, Petty A, Craig JP. Ocular surface microbiome in meibomian gland dysfunction in Auckland, New Zealand. Clin Exp Ophthalmol 2016.
- ↑ Tomlinson A, Bron AJ, Korb DR, et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Diagnosis Subcommittee. Investigative Ophthalmology & Visual Science 2011;52:2006-49.
- ↑ Napoli PE, Coronella F, Satta GM, Iovino C, Sanna R, Fossarello M. A Simple Novel Technique of Infrared Meibography by Means of Spectral-Domain Optical Coherence Tomography: A Cross-Sectional Clinical Study. PLoS One 2016;11:e0165558.
- ↑ Fasanella V, Agnifili L, Mastropasqua R, et al. In Vivo Laser Scanning Confocal Microscopy of Human Meibomian Glands in Aging and Ocular Surface Diseases. Biomed Res Int 2016;2016:7432131.
- ↑ Liang QF, Gao C, Liang H, Du XH, Wang NL, Labbe A. [In vivo confocal microscopy evaluation of meibomian glands in meibomian gland dysfunction patients]. Zhonghua Yan Ke Za Zhi 2016;52:649-56.
- ↑ Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2011;52:2050-64.
- ↑ Wladis EJ, Aakalu VK, Foster JA, Freitag SK, Sobel RK, Tao JP, Yen MT. Intense Pulsed Light for Meibomian Gland Disease: A Report by the American Academy of Ophthalmology. Ophthalmology. 2020 Sep;127(9):1227-1233. doi: 10.1016/j.ophtha.2020.03.009. Epub 2020 Apr 21. PMID: 32327256.
- ↑ reiner JV. A single LipiFlow(R) Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res 2012;37:272-8.
- ↑ Zhao Y, Veerappan A, Yeo S, et al. Clinical Trial of Thermal Pulsation (LipiFlow) in Meibomian Gland Dysfunction With Preteatment Meibography. Eye Contact Lens 2016;42:339-46.
- ↑ Nam S, Lim DH, Hyun J, Chung T-Y. Evaluation of KCL 1100® Automated Thermodynamic System Treatment for Dry Eye with Meibomian Gland Dysfunction. Investigative Ophthalmology & Visual Science 2016;57:5679.
- ↑ Connor CG, Choat C, Narayanan S, Kyser K, Rosenberg B, Mulder D. Clinical Effectiveness of Lid Debridement with BlephEx Treatment. Investigative Ophthalmology & Visual Science 2015;56:4440-.
- ↑ Yeo S, Tan JH, Acharya UR, Sudarshan VK, Tong L. Longitudinal Changes in Tear Evaporation Rates After Eyelid Warming Therapies in Meibomian Gland DysfunctionTear Evaporation After Eyelid Warming. Investigative Ophthalmology & Visual Science 2016;57:1974-81.
- ↑ Jackson C, Tashbayev B, Ræder S, et al. Treatment Effects in a Norwegian Cohort of Dry Eye Patients with Meibomian Gland Dysfunction Following Treatment According to Guidelines of The International Workshop on Meibomian Gland Dysfunction (2011): Three and Six Months Follow-up. Investigative Ophthalmology & Visual Science 2016;57:5665.
- ↑ Kashkouli MB, Fazel AJ, Kiavash V, Nojomi M, Ghiasian L. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomized double-masked open-label clinical trial. Br J Ophthalmol. 2015 Feb;99(2):199-204. doi: 10.1136/bjophthalmol-2014-305410. Epub 2014 Aug 19. PMID: 25138765.