Kearns-Sayre Ptosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Kearns-Sayre syndrome is a mitochondrial myopathy with systemic and ocular manifestations including cardiac conduction defects, pigmentary retinopathy and chronic progressive external ophthalmoplegia.
Disease Entity
Kearns-Sayre Syndrome ICD-10 H49.81
Disease
The syndrome was first described in 1958 when Kearns and Sayre presented two patients with a triad of chronic progressive external ophthalmoplegia, pigmentary retinopathy and cardiac conduction defects leading to heart block.[2] The syndrome belongs to a diverse group of mitochondrial myopathies characterized by the presence of “ragged red fibers” seen in Trichrome stained skeletal muscle biopsies.[3] The mitochondrial dysfunction seen in Kearns-Sayre syndrome results in a wide spectrum of systemic manifestations including ocular, cardiac, endocrine and neurological abnormalities. The ocular sequelae, in particular bilateral ptosis and ophthalmoplegia, are often the presenting signs of the disease.
Etiology
Kearns-Sayre syndrome is a genetic disorder most commonly due to single sporadic deletions in mitochondrial DNA. However, the clinical phenotype associated with Kearns-Sayre syndrome can result from deletions, duplications, rearrangements or mutations of nuclear or mitochondrial DNA that encodes proteins or enzymes necessary for oxidative phosphorylation.
Risk Factors
There is no apparent sex predilection and there are no known risk factors.
Prevalence and Incidence
Over 200 cases of Kearns-Sayre syndrome have been published, but the true incidence of the disease remains unknown.
General Pathology
Skeletal muscle biopsy stained with Massons or Gomori trichrome exhibits “ragged red fibers” resulting from increased numbers of enlarged sub-sarcolemmal mitochondria (Figure 1).[4] Electron microscopy reveals increased numbers of abnormal mitochondria. The mitochondria are often enlarged, with disorganized cristae and paracrystalline inclusions. Retinal evaluation reveals disruption and atrophy of retinal photoreceptors as well as aberrant pigment distribution in all layers of the retina.[5][6] Spongiform degeneration of the brain has been reported in multiple patients at autopsy.[7]
Pathophysiology
Kearns-Sayre syndrome results from alterations in mitochondrial or nuclear DNA that disrupt oxidative phosphorylation, impairing ATP production in affected mitochondria. The manifestations vary depending on the degree of mitochondrial dysfunction and types of tissues affected. Organ dysfunction may occur by a number of mechanisms.
Genetics
The majority of patients with Kearns-Sayre syndrome have a sporadic deletion of mitochondrial DNA, although nuclear DNA defects disrupting oxidative phosphorylation have also been identified. Mitochondrial DNA exists as double-stranded circle of approximately 16,500 base pairs, inherited via maternal mitochondria present in the oocyte at the time of fertilization. Mitochondrial DNA defects cause disease by disrupting regions that code for electron transport chain subunits. Due to a phenomenon known as heteroplasmy, the percentage of abnormal mtDNA copies in any given mitochondrion can vary. In addition, each time a cell replicates there is a random assignment of mitochondria to each of the daughter cells. As a result, some cells by chance receive greater numbers of abnormal mitochondria than others. Over time the proportion of abnormal mtDNA is thought to increase due to a slightly greater success rate in replicating abnormal mitochondrial DNA transcripts within each mitochondrion. The preferential replication of the abnormal mtDNA is thought to occur because the relatively shorter length of the abnormal transcripts allows them to replicate faster than full-length mtDNA.[8] Although the relative proportion of abnormal mtDNA is highest in skeletal muscle, a direct link between disease severity and proportion of abnormal mtDNA has not been firmly established.[9]
In addition to mitochondrial defects, nuclear DNA defects cause disease by disrupting regions coding for electron transport chain subunits or enzymes essential to oxidative phosphorylation. The nuclear DNA abnormalities disrupt polymerases involved in the replication of mtDNA and proteins involved in the transport and metabolism of substrates necessary for mtDNA synthesis.[10]
Due to the potential for defects in either mitochondrial or nuclear DNA defects, the inheritance pattern can be maternal, autosomal recessive or autosomal dominant.[11][12]
Diagnosis
History
Patients with Kearns-Sayre syndrome typically present within the first two decades of life with signs of and symptoms due to bilateral, progressive ptosis and external ophthalmoplegia. Most cases are sporadic with a negative family history, although inherited forms have been described. Serial examination of old photos can be invaluable in establishing the chronic, progressive nature of the disease. Although the ophthalmoplegia may be less severe than the ptosis at presentation, it usually becomes progressively worse over a period of years. The systemic manifestations typically follow a similar pattern of chronic progressive worsening, in particular the cardiac conduction defects ultimate result in complete heart block, which has been described up to 8 years after diagnosis.[13] Despite the presence of retinopathy, visual acuity is often preserved, although severe macular involvement and poor vision have been described.[14]
Physical Examination
A thorough evaluation of extraocular movements including ductions, versions and levator function is essential in identifying the extent of the ophthalmoplegia and the etiology of the ptosis. Slit-lamp examination of the anterior segment is typically normal. Dilated fundus examination is important to identify pigmentary retinopathy and rarely, optic neuritis.[15] Due to the systemic nature of the disease, physical examination focusing on neurological, cardiac, auditory and endocrine systems may help establish the diagnosis.
Signs
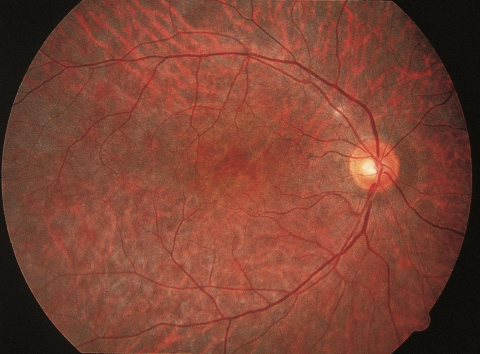
Bilateral, symmetrical ptosis and ophthalmoplegia are critical early signs. In more advanced ophthalmoplegia, patients may turn the entire head or body to view objects outside of primary gaze. The ptosis is characterized by decreased levator function as there is an inherent abnormality in the muscle itself but not the dehiscence of the levator aponeurosis. Due to the slow, symmetrical onset of ophthalmoplegia, most patients do not experience diplopia until late in their course or not at all. The pigmentary retinopathy can range from mild to severe, is more prominent in the later stages of the disease and often presents with a diffuse stippled “salt and pepper” appearance involving the macula and midperiphery, as well as peripapillary retinal epithelial atrophy.[2][3][5][16][17] Cardiac auscultation can identify rate or rhythm abnormalities due to heart block and rarely, murmurs related to mitral valve regurgitation.[13] Hearing loss, cerebellar ataxia and cognitive impairment are also potential diagnostic signs.[2][17] Skeletal muscle weakness can result in myopathic facies and may progress to include wasting of the neck and shoulder muscles and even mild limb weakness.[2][11][17][18] Up to 38% of patients may exhibit short stature.[19] Endocrine dysfunction can result in gonadal dysfunction, hyperglycemia, hypocalcemia and hyperphosphatemia. [19][20][21][22][23][24]
Symptoms
Patients often present with complaints of drooping eyelids. Diplopia can result from asymmetric progression of the chronic external ophthalmoplegia. Visual symptoms related to acuity may occur in conjunction with progression of pigmentary retinopathy, but most patients have relatively good vision at the time of diagnosis. Despite findings of proximal muscle weakness documented in some Kearns-Sayre patients, they may be unaware of or deny noticeable weakness.[18]
Clinical diagnosis
The criteria for diagnosis are onset of CPEO and pigmentary retinopathy prior to age 20, plus any one of the following: complete heart block, CSF protein > 1mg/ml, cerebellar ataxia, short stature, hearing loss, dementia and endocrine abnormalities.[3]
Diagnostic procedures
Biopsy and Genetic Testing
Biopsy of skeletal muscle can be helpful in establishing the diagnosis. Although the presence of ragged red fibers on biopsy is not part of the current diagnostic criteria, it both supports the diagnosis and provides a tissue sample for genetic testing. Due to mitochondrial heteroplasmy and the potential uneven distribution of the genetic defect among different tissue types, biopsy is usually taken from an affected skeletal muscle.
Genetic testing should be coordinated with a laboratory familiar with mitochondrial diseases. The wide variety of genetic defects associated with the disease may necessitate extensive genetic testing and this should be considered when obtaining biopsies and anticipating the potential expense of obtaining a definitive genetic diagnosis.
Electroretinogram
Electroretinography and dark adaptometry are usually normal or only mildly abnormal.[11][25]
Electrocardiogram
All patients with suspected Kearns-Sayre syndrome should be referred to a primary physician or cardiologist to have an electrocardiogram to screen for heart block. A subset of patients with Kearns-Sayre syndrome may have mitral valve regurgitation and an echocardiogram may be warranted based on clinical examination and consultation with a cardiologist.[13]
Endocrine Evaluation
Endocrine dysfunction is common and should be evaluated in conjunction with an endocrine specialist. Hypoparathyroidism, diabetes mellitus, Addison’s disease and growth hormone deficiency have all been reported.[19][20][21][22][23][24]
CSF Analysis
CSF protein greater than 1mg/ml is a potential diagnostic criterion. Elevated CSF pyruvate and lactate have also been reported.[26]
Auditory Testing
Auditory testing can be useful for characterizing hearing loss associated with Kearns-Sayre syndrome.
Imaging
Fluorescein angiography demonstrates retinal pigment mottling with areas of hyper and hypofluorescence as a result of interspersed retinal pigment clumping and atrophy primarily within the posterior pole. Increased choroidal fluorescence can be seen in areas of RPE atrophy.[16][27] MRI of the brain may reveal progressive lesions involving the brainstem, globus pallidus, thalamus and white matter of the cerebrum and cerebellum.[28]
Differential diagnosis
The differential diagnosis includes mitochondrial cytopathies that can have both genetic and phenotypic overlap with Kearns-Sayre syndrome. These include MELAS (mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes), MERRF (myoclonic epilepsy with ragged red fibers), NARP (neuropathy, ataxia and retinitis pigmentosa), Pearson syndrome (characterized by marrow failure, pancreatic dysfunction, lactic acidosis) and isolated chronic progressive external ophthalmoplegia. Myasthenia gravis and myotonic dystrophy should also be considered.
Management
Global
Recent studies have shown beneficial results with coenzyme Q10 supplementation in cardiac conduction, ocular motility, and corneal edema. The combination of coenzyme Q10 with creatine monohydrate and alpha-lipoid acid showed improvement in oxidative stress markers in mitochondrial diseases [29] Furthermore, there are clinical trials evaluating the effect of a vitamin E-derived coenzyme Q10 analogue (ClinicalTrials.gov #NCT01370447) and low carbohydrate/high-fat diets (ClinicalTrials.gov #NCT02385565) in mitochondrial disorders including Kearns-Sayre syndrome.
Ocular
There is no definitive treatment for Kearns-Sayre syndrome although symptomatic and preventative treatments can improve quality of life. Ptosis can be surgically corrected with a frontalis suspension, although the efficacy of the procedure may wane in the later stages of the disease if the facial muscles become involved. Often ptosis recurs or progresses, requiring additional upper eyelid surgery. Patients with diplopia may benefit from prisms or strabismus surgery.
Cardiac
Cardiac dysfunction due to progressive arrhythmia can lead to stroke or death and evaluation by a cardiologist at the time of diagnosis and at regular intervals thereafter is suggested. Conduction abnormalities have been successfully managed with pacemaker implantation.[7] In cases of severe cardiac dysfunction, heart transplant is an option.[30] Monitoring with annual ECG, 2D-echocardiography, 24-hour Holter monitoring and endocrine assessment are important.
Prognosis
The visual prognosis for Kearns-Sayre syndrome is relatively good. Only 40-50% of patients develop mild visual acuity disturbance or night blindness and central vision is usually preserved throughout life.[3][14][16] Mortality depends largely on the severity of cardiac disease, although endocrine dysfunction has also been a cause of significant morbidity and mortality in Kearns-Sayre patients.
Summary
Kearns-Sayre syndrome is a mitochondrial disease that presents with progressive ptosis and extraocular muscle restriction in a young adult. Care should be taken to evaluate these patients for systemic disease including cardiac conduction deficits.
References
- ↑ American Academy of Ophthalmology. Kearns-Sayre syndrome. https://www.aao.org/image/kearns-sayre-syndrome Accessed July 08, 2019.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Kearns TP, Sayre GP. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch Ophthalmol. Aug 1958;60(2):280-289.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Berenberg RA, Pellock JM, DiMauro S, et al. Lumping or splitting? "Ophthalmoplegia-plus" or Kearns-Sayre syndrome? Ann Neurol. Jan 1977;1(1):37-54.
- ↑ Karpati G, Carpenter S, Larbrisseau A, Lafontaine R. The Kearns-Shy syndrome. A multisystem disease with mitochondrial abnormality demonstrated in skeletal muscle and skin. J Neurol Sci. Jun 1973;19(2):133-151.
- ↑ Jump up to: 5.0 5.1 Eagle RC, Jr., Hedges TR, Yanoff M. The atypical pigmentary retinopathy of Kearns-Sayre syndrome. A light and electron microscopic study. Ophthalmology. Dec 1982;89(12):1433-1440.
- ↑ Eagle RC, Jr., Hedges TR, Yanoff M. The Kearns-Sayre syndrome: a light and electron microscopic study. Trans Am Ophthalmol Soc. 1982;80:218-234.
- ↑ Jump up to: 7.0 7.1 Drachman DA. Ophthalmoplegia plus. The neurodegenerative disorders associated with progressive external ophthalmoplegia. Arch Neurol. Jun 1968;18(6):654-674.
- ↑ Brown MD, Wallace DC. Molecular basis of mitochondrial DNA disease. J Bioenerg Biomembr. Jun 1994;26(3):273-289.
- ↑ Moraes CT, DiMauro S, Zeviani M, et al. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. May 18 1989;320(20):1293-1299.
- ↑ McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. Aug 2010;9(8):829-840.
- ↑ Jump up to: 11.0 11.1 11.2 Leveille AS, Newell FW. Autosomal dominant Kearns-Sayre syndrome. Ophthalmology. Feb 1980;87(2):99-108.
- ↑ Nemet P, Godel V, Lazar M. Kearns-Sayre syndrome. Birth Defects Orig Artic Ser. 1982;18(6):263-268.
- ↑ Jump up to: 13.0 13.1 13.2 Darsee JR, Miklozek CL, Heymsfield SB, Hopkins LC, Jr., Wenger NK. Mitral valve prolapse and ophthalmoplegia: a progressive, cardioneurologic syndrome. Ann Intern Med. Jun 1980;92(6):735-741.
- ↑ Jump up to: 14.0 14.1 Mullie MA, Harding AE, Petty RK, Ikeda H, Morgan-Hughes JA, Sanders MD. The retinal manifestations of mitochondrial myopathy. A study of 22 cases. Arch Ophthalmol. Dec 1985;103(12):1825-1830.
- ↑ Davis JC, Reiffel JA, Behrens M, Rowland L, Mascitelli R, Seplowitz A. Optic neuritis and heart block in Kearns-Sayre syndrome. N Y State J Med. Aug 1981;81(9):1364-1368.
- ↑ Jump up to: 16.0 16.1 16.2 Gross-Jendroska M, Schatz H, McDonald HR, Johnson RN. Kearns-Sayre syndrome: a case report and review. Eur J Ophthalmol. Jan-Mar 1992;2(1):15-20.
- ↑ Jump up to: 17.0 17.1 17.2 Kearns TP. External Ophthalmoplegia, Pigmentary Degeneration of the Retina, and Cardiomyopathy: A Newly Recognized Syndrome. Trans Am Ophthalmol Soc. 1965;63:559-625.
- ↑ Jump up to: 18.0 18.1 Miller NR, Walsh FB, Hoyt WF, Ovid Technologies Inc. Walsh and Hoyt's clinical neuro-ophthalmology. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2005.
- ↑ Jump up to: 19.0 19.1 19.2 Harvey JN, Barnett D. Endocrine dysfunction in Kearns-Sayre syndrome. Clin Endocrinol (Oxf). Jul 1992;37(1):97-103.
- ↑ Jump up to: 20.0 20.1 Bachynski BN, Flynn JT, Rodrigues MM, Rosenthal S, Cullen R, Curless RG. Hyperglycemic acidotic coma and death in Kearns-Sayre syndrome. Ophthalmology. Mar 1986;93(3):391-396.
- ↑ Jump up to: 21.0 21.1 Horwitz SJ, Roessmann U. Kearns-Sayre syndrome with hypoparathyroidism. Ann Neurol. Jun 1978;3(6):513-518.
- ↑ Jump up to: 22.0 22.1 Pellock JM, Behrens M, Lewis L, Holub D, Carter S, Rowland LP. Kearns-Sayre syndrome and hypoparathyroidism. Ann Neurol. May 1978;3(5):455-458.
- ↑ Jump up to: 23.0 23.1 Burns EC, Preece MA, Cameron N, Tanner JM. Growth hormone deficiency in mitochondrial cytopathy. Acta Paediatr Scand. Jul 1982;71(4):693-697.
- ↑ Jump up to: 24.0 24.1 Obara-Moszynska M, Maceluch J, Bobkowski W, et al. A novel mitochondrial DNA deletion in a patient with Kearns-Sayre syndrome: a late-onset of the fatal cardiac conduction deficit and cardiomyopathy accompanying long-term rGH treatment. BMC Pediatr. 2013;13:27.
- ↑ Koerner F, Schlote W. Chronic progressive external ophthalmoplegia: association with retinal pigmentary changes and evidence in favor of ocular myopathy. Arch Ophthalmol. Aug 1972;88(2):155-166.
- ↑ Kuriyama M, Suehara M, Marume N, Osame M, Igata A. High CSF lactate and pyruvate content in Kearns-Sayre syndrome. Neurology. Feb 1984;34(2):253-255.
- ↑ Lowes M. Chronic progressive external ophthalmoplegia, pigmentary retinopathy, and heart block (Kearns-Sayre syndrome). Report of a case. Acta Ophthalmol (Copenh). Sep 1975;53(4):610-619.
- ↑ Nakagawa E, Hirano S, Yamanouchi H, Goto Y, Nonaka I, Takashima S. Progressive brainstem and white matter lesions in Kearns-Sayre syndrome: a case report. Brain Dev. Sep-Oct 1994;16(5):416-418.
- ↑ Tarnopolsky MA. The mitochondrial cocktail: rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv. Drug Deliv Rev. 2008;60:1561-7.
- ↑ Tranchant C, Mousson B, Mohr M, et al. Cardiac transplantation in an incomplete Kearns-Sayre syndrome with mitochondrial DNA deletion. Neuromuscul Disord. Sep-Nov 1993;3(5-6):561-566.