Herpes Simplex Virus Stromal Keratitis and Endotheliitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Herpes Simplex Virus (HSV) Stromal Keratitis and Endotheliitis
Disease
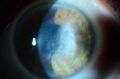
HSV infection can cause inflammation in nearly every ocular tissue. In cases of corneal involvement, the epithelium, stroma, or endothelium may be affected. Both herpes stromal keratitis (HSK) and HSV endotheliitis can present clinically with stromal opacity and, therefore, may be difficult to distinguish. In HSK, stromal opacity is due to immunopathology within the stroma, is often associated with corneal neovascularization, and recurrent episodes can lead to irreversible stromal scarring and vision loss.
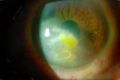
In HSV endotheliitis, either secondary inflammation caused by the virus and/or direct infection of endothelial cells is thought to cause endothelial dysfunction and subsequent stromal edema and opacity. Stromal scarring from true endotheliitis is rare. While presentation may appear similar between HSK and HSV endotheliitis, they represent two distinct pathologic mechanisms that are both induced by HSV infection.
Etiology
The majority of ocular HSV infections are caused by HSV type 1 (HSV-1), except in cases of neonatal ocular infections, which are largely caused by HSV-2 contracted during decent through an infected birth canal.[1] The herpes viruses are unique in their ability to cause productive infections as well as establish latency, a relatively quiescent state in which the viral genome is retained in the absence of productive virus particle production. Therefore, the virus is never completely eradicated from the host and may reactivate causing recurrent disease. In response to certain stressors, including exposure to ultraviolet light,[2] psychological stress [3], and hormonal fluctuation, [4] HSV may reactivate from latency to produce infectious virions with recurrent disease at the initial sight of infection. HSV infection of the mucosal and epithelial surfaces of the orofacial region, including the cornea, precedes retrograde axonal transport of the virus within the respective division of the trigeminal nerve to the trigeminal ganglia, where additional viral replication occurs and latency is established within neuronal nuclei. In times of stress, the virus may reactivate, travel back down the ophthalmic division of the trigeminal nerve and cause recurrent disease in the cornea. It has also been suggested that the cornea itself may be a reservoir for viral latency, although this theory has yet to be definitively proven.[5]
Epidemiology
HSV-1 infects the majority of the world’s adult population with recent seroprevalence studies from the United States and Germany detecting 57.7% and ~75% HSV-1 positivity, respectively.[6] [7] The annual incidence of ocular HSV infections has recently been estimated at 11.8 per 100000 people in the United States[8] and 13.2 per 100000 in France, where the incidence of recurrence was 18.3 per 100000 people. [9] In the Herpetic Eye Disease Study, 18% of patients diagnosed with HSV-1 ocular disease experienced a recurrence involving the stroma,[10] and stromal keratitis represented 44% of all recurrences.[11] Furthermore, previous bouts of HSK significantly increased the risk of future recurrences of stromal disease. Therefore, HSK represents a significant burden of ocular disease caused by HSV-1 infection.
The epidemiology of HSV endotheliitis is less clear at least in part because many publications include disciform HSV endotheliitis as a form of HSK.
Clinical manifestations
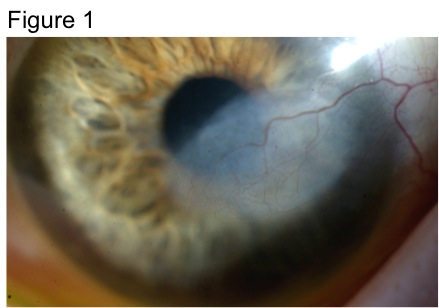
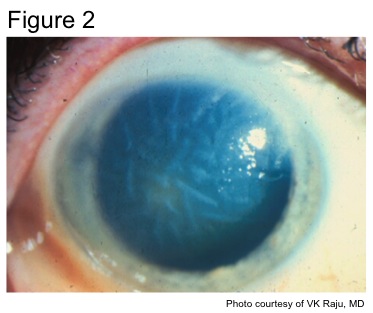
While both HSK and HSV endotheliitis can present with stromal opacity, they are separate pathological processes. The stromal opacity in HSK is driven by inflammation within the stroma that is often accompanied by neovascularization (Figure 1). The opacity in HSV endotheliitis is the result of stromal edema due to HSV-mediated endothelial dysfunction in the absence of stromal inflammation or neovascularization (Figure 2).
HSK can be classified as either necrotizing or non-necrotizing.[12] [13] In necrotizing HSK, an overlying epithelial defect is often present, and the risk of stromal melting and perforation is high. Both viral and immune-mediated destruction of the cornea is implicated in necrotizing HSK. Conversely, in non-necrotizing HSK, also known as immune or interstitial HSK, the epithelium is intact, and the pathology is thought to driven primarily by the host immune response.
In HSV endotheliitis, stromal edema is often accompanied by underlying keratic precipitates (KP) and an anterior chamber inflammatory reaction. HSV endotheliitis is categorized into three main forms, namely disciform, which is the most common, diffuse, and linear, based on the pattern of endothelial dysfunction and stromal edema. Intraocular pressure may be elevated secondary to trabeculitis. [14]
Pathophysiology
The pathophysiology of HSK is complex and remains incompletely understood. Current data suggests that CD4 T cells of the adaptive immune system are required for the development and maintenance of HSK. [15]
Based on extensive studies in animal models, several theories exist as to the stimulus for the immunopathological CD4 T cell response:
- CD4 T cells are HSV-specific and driven by the presence of HSV antigen within the stroma;[16]
- The inflammatory milieu created by HSV corneal infection drives a non-specific bystander CD4 T cell response;[17]
- Autoreactive CD4 T cells are stimulated by corneal proteins that mimic HSV proteins, [18] although this observation has not been reproduced and likely represents a phenomenon specific to the animal model used in the study
- Or a combination of these mechanisms.
HSV DNA, protein, or live virus is inconsistently found in cases of HSK, in comparison to HSV epithelial keratitis where HSV is often identified. Other leukocytes, such as neutrophils, also infiltrate the stroma during HSK, and stromal neovascularization is a key component of the inflammatory cascade that leads to stromal opacity.[19]
[20]
Deturgesence of the corneal stroma is accomplished in part by the pump mechanism of the endothelium. Endothelial cell dysfunction can lead to inadequate stromal dehydration with subsequent edema and opacity. In HSV endotheliitis, endothelial cells are dysfunctional due to either direct infection with HSV or as a consequence of the anterior chamber inflammation induced by HSV infection. Inflammation with the stoma can be a component of HSV endotheliitis.
Diagnosis
The diagnosis of HSK and HSV endotheliitis is predominantly clinical, based on a history of recurrent herpetic ocular disease and slit lamp examination of the eye revealing characteristic herpetic lesions as described above. Additionally, PCR analysis, enzyme-linked assays, and viral culture of the tear film may reveal HSV-1 DNA, protein, or live virus, respectively.[21] However, sensitivity of these tests is greatly reduced in the absence of active corneal ulceration, such as in non-necrotizing HSK and HSV endotheliitis.[22]While virus has been isolated from the anterior chamber of patients with HSV endotheliitis, it is rarely detected in the tear film.[23]
Differential diagnosis
The differential diagnosis for HSK includes other etiologies of infectious interstitial keratitis, such as other viruses (Varicella zoster virus, Epstein-Barr virus), bacteria (syphilis, lyme), fungus, and Acanthamoeba, as well as immune-mediated etiologies, including sarcoidosis and Cogan syndrome.
The differential diagnosis for HSV endotheliitis includes endothelial dysfunction caused by other herpes viruses (Varicella zoster virus, cytomegalovirus) as well as the inflammatory glaucomatous conditions Fuchs’ heterochromic iridocyclitis and Posner-Schlossman syndrome, which have been postulated to have viral etiologies, including herpes viruses.[24] [25] [26]
Management
Current management of acute HSK and HSV endotheliitis is aimed at inhibiting viral replication with oral or topical antiviral medications and reducing inflammatory damage with topical corticosteroids. The Herpetic Eye Disease Study (HEDS) was a series of randomized placebo-controlled clinical trials initiated in 1989 that set the treatment guidelines for HSV keratitis and iridocyclitis.
Medical therapy
As discussed above, the immune response to corneal HSV-1 infection is a major contributor to the stromal damage and subsequent scarring responsible for the blinding complications of HSK. HEDS established the benefit of topical corticosteroids in the treatment of HSV stromal keratitis. Topical corticosteroids are often utilized in combination with oral antiviral medication to block potential viral replication [27]
Surgical therapy
In cases of chronic or recurrent HSK in which stromal scarring has caused severe visual impairment, corneal transplantation in the form of either full-thickness penetrating keratoplasty or anterior lamellar keratoplasty may be attempted to restore corneal clarity. It is well established that full-thickness grafts placed in patients with a history of HSK have a higher rate and incidence of rejection compared to grafts placed for non-inflammatory conditions.[28]
The exact mechanisms responsible for accelerated graft rejection in patients with a history of herpetic eye disease remains incompletely understood but viral reactivation induced by the grafting procedure with severing of trigeminal nerve termini is thought to contribute to the rejection process. Thus, it is common practice to place patients undergoing corneal transplantation for HSV corneal infection on long-term systemic antiviral therapy to inhibit potential viral replication.
More recently, deep anterior lamellar keratoplasty (DALK) has been used as an alternative surgical approach in patients with visually significant stromal scars from HSK in which the endothelium remains healthy. DALK offers the option of replacing only the corneal stroma with preservation of the host endothelium, which is often the focus of transplant rejection. Initial results using DALK appear promising with several small studies demonstrating high rates of graft survival.[29] [30] [31]
Prophylactic therapy
The potentially blinding complications of HSK stem from the recurrent nature of the disease. Repeated bouts of stromal inflammation may eventually lead to irreversible scarring and loss of sight. Recurrences are likely driven by viral reactivation in the trigeminal ganglion with transport of the virus to the cornea, leading to a local immune response within the stroma. A potentially useful mechanism of therapy, therefore, involves inhibiting viral reactivation to prevent recurrent corneal inflammation. A landmark trial that was part of the Herpetic Eye Disease Study demonstrated that systemic antiviral therapy with oral acyclovir significantly reduced the frequency of recurrent ocular HSV-1 disease.[32] In patients with a history of stromal disease specifically, oral acyclovir treatment reduced the probability of HSK recurrence by 50%, from 28% to 14%. These data suggest that blocking viral replication systemically, likely within the trigeminal ganglia where reactivation begins, inhibits the impetus for recurrent stromal inflammation. Moreover, acyclovir’s mechanism of action, phosphorylation by viral thymidine kinase with subsequent incorporation into viral DNA by viral DNA polymerase resulting in strand termination, requires active viral DNA replication, suggesting that HSV-1 must be at least in the process of reactivating for acyclovir to function.
Current research
Current research has investigated therapeutic HSV vaccination strategies with the goal of inhibiting viral reactivation within the trigeminal ganglia and preventing reactivation events. CD8 T cells of the adaptive immune response function to inhibit viral reactivation within the trigeminal ganglion using non-lytic mechanisms.[33] Therefore, a therapeutic vaccine designed to boost the CD8 T cell response within latently infected ganglia may achieve inhibition of viral reactivation and subsequent corneal inflammation without the need for daily oral medication. Consistent with this hypothesis, a small controlled trial in humans demonstrated a significant reduction in HSV-1 ocular recurrences following subcutaneous administration of heat-inactivated HSV-1.[34] Although there was a trend toward decreased HSK recurrences in the vaccinated group, the study was not powered to achieve statistical significance for this subgroup of HSV ocular disease. Larger clinical trials are required to validate this finding.
References
- ↑ Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol 2006;141(3):547-57.
- ↑ Spurney RV, Rosenthal MS. Ultraviolet-induced recurrent herpes simplex virus keratitis. Am J Ophthalmol 1972;73(4):609-10.
- ↑ Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol 2007;179(1):322-8.
- ↑ Cherpes TL, Busch JL, Sheridan BS, et al. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol 2008;181(2):969-75.
- ↑ Kennedy DP, Clement C, Arceneaux RL, et al. Ocular herpes simplex virus type 1: Is the cornea a reservoir for viral latency or a fast pit stop? Cornea 2011;30(3):251-9.
- ↑ Rabenau HF, Buxbaum S, Preiser W, et al. Seroprevalence of herpes simplex virus types 1 and type 2 in the Frankfurt am Main area, Germany. Med Microbiol Immunol 2002;190(4):153- 60.
- ↑ Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006;296(8):964-73.
- ↑ Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: The effect of oral antiviral prophylaxis. Arch Ophthalmol 2010;128(9):1178-83.
- ↑ Labetoulle M, Auquier P, Conrad H, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmol 2005;112(5):888-95.
- ↑ Predictors of recurrent herpes simplex virus keratitis. Herpetic Eye Disease Study Group. Cornea 2001;20(2):123-8.
- ↑ Oral acyclovir for herpes simplex virus eye disease: Effect on prevention of epithelial keratitis and stromal keratitis. Herpetic Eye Disease Study Group. Arch Ophthalmol 2000;118(8):1030-6.
- ↑ Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea 1999;18(2):144-54.
- ↑ Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea 1999;18(2):127-43.
- ↑ Amano S, Oshika T, Kaji Y, et al. Herpes simplex virus in the trabeculum of an eye with corneal endotheliitis. Am J Ophthalmol 1999;127(6):721-2.
- ↑ Knickelbein JE, Beula KA, Hendricks RL. Herpes stromal keratitis: Erosion of ocular immune privilege by herpes simplex virus. Future Virol 2010;5(6):699-708.
- ↑ Verjans GM, Remeijer L, van Binnendijk RS, et al. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect Dis 1998;177(2):484-8.
- ↑ Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Invest Ophthalmol Vis Sci 2000;41(2):453-9.
- ↑ Zhao ZS, Granucci F, Yeh L, et al. Molecular mimicry by herpes simplex virus-type 1: Autoimmune disease after viral infection. Science 1998;279(5355):1344-7.
- ↑ Rowe AM, St Leger AJ, Jeon S, et al. Herpes keratitis. Prog Retin Eye Res 2012; Epub ahead of print.
- ↑ Gimenez F, Suryawanshi A, Rouse BT. Pathogenesis of herpes stromal keratitis - A focus on corneal neovascularization. Prog Retin Eye Res 2012; Epub ahead of print.
- ↑ Kowalski RP, Gordon YJ, Romanowski EG, et al. A comparison of enzyme immunoassay and polymerase chain reaction with the clinical examination for diagnosing ocular herpetic disease. Ophthalmol 1993;100(4):530-3.
- ↑ Fukuda M, Deai T, Higaki S, et al. Presence of a large amount of herpes simplex virus genome in tear fluid of herpetic stromal keratitis and persistent epithelial defect patients. Semin Ophthalmol 2008;23(4):217-20.
- ↑ Fukuda M, Deai T, Hibino T, et al. Quantitative analysis of herpes simplex virus genome in tears from patients with herpetic keratitis. Cornea 2003;22(7 Suppl):60.
- ↑ de Groot-Mijnes JD, de Visser L, Rothova A, et al. Rubella virus is associated with fuchs heterochromic iridocyclitis. Am J Ophthalmol 2006;141(1):212-4.
- ↑ Barequet IS, Li Q, Wang Y, et al. Herpes simplex virus DNA identification from aqueous fluid in Fuchs heterochromic iridocyclitis. Am J Ophthalmol 2000;129(5):672-3.
- ↑ Yamamoto S, Pavan-Langston D, Tada R, et al. Possible role of herpes simplex virus in the origin of Posner-Schlossman syndrome. Am J Ophthalmol 1995;119(6):796-8.
- ↑ Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: An evidence-based review. Surv Ophthalmol 2009;54(2):226-34.
- ↑ Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol 2005;140(6):1112-22.
- ↑ Sarnicola V, Toro P. Deep anterior lamellar keratoplasty in herpes simplex corneal opacities. Cornea 2010;29(1):60-4.
- ↑ Wang J, Zhao G, Xie L, et al. Therapeutic effect of deep anterior lamellar keratoplasty for active or quiescent herpetic stromal keratitis. Graefes Arch Clin Exp Ophthalmol 2012;250(8):1187-94.
- ↑ Wu SQ, Zhou P, Zhang B, et al. Long-term comparison of full-bed deep lamellar keratoplasty with penetrating keratoplasty in treating corneal leucoma caused by herpes simplex keratitis. Am J Ophthalmol 2012;153(2):291-9 e2.
- ↑ Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. NEJM 1998;339(5):300-6.
- ↑ Knickelbein JE, Khanna KM, Yee MB, et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 2008;322(5899):268-71.
- ↑ Pivetti-Pezzi P, Accorinti M, Colabelli-Gisoldi RA, et al. Herpes simplex virus vaccine in recurrent herpetic ocular infection. Cornea 1999;18(1):47-51.