Gyrate Atrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Gyrate Atrophy of the choroid and the retina is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
ICD-10 H31.23
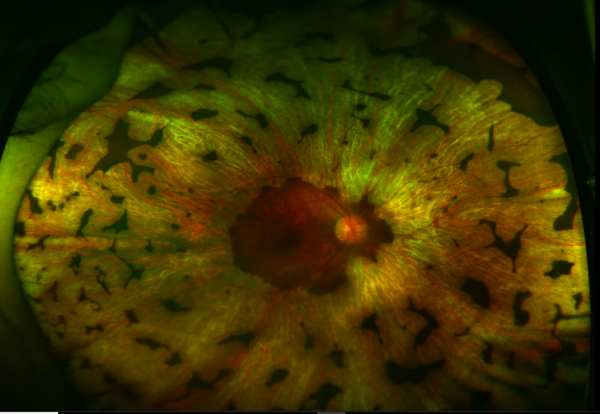
Disease
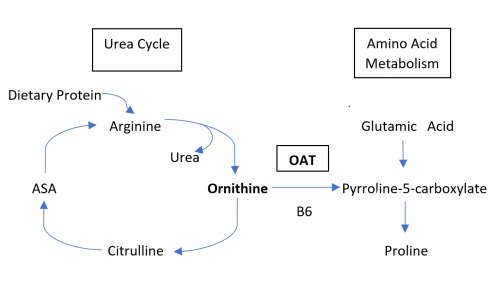
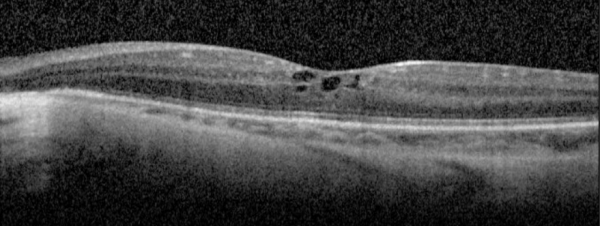
Gyrate Atrophy of the choroida and the retina is a rare autosomal recessive retinal dystrophy characterized by progressive chorioretinal degeneration, early cataract formation and myopia. It is caused by a deficiency in the enzyme ornithine aminotransferase (OAT), which results in a 10- to 20-fold increase in plasma ornithine concentrations. Patients classically present in the first decade of life with nyctalopia, with fundus exam revealing characteristic circular patches of chorioretinal atrophy distributed in the peripheral fundus (Figure 1). As the disease progresses, the atrophic lesions coalesce and advance centripetally toward the posterior pole[1] (Figure 2), correlating with progressive loss of peripheral vision. Macular involvement occurs late in the disease.
Prevalence and Incidence
Approximately 200 biochemically confirmed cases have been reported in the literature. The highest rate is in Finland with one case per 50,000 individuals. [2] There is no predilection for gender. [3]
Genetics
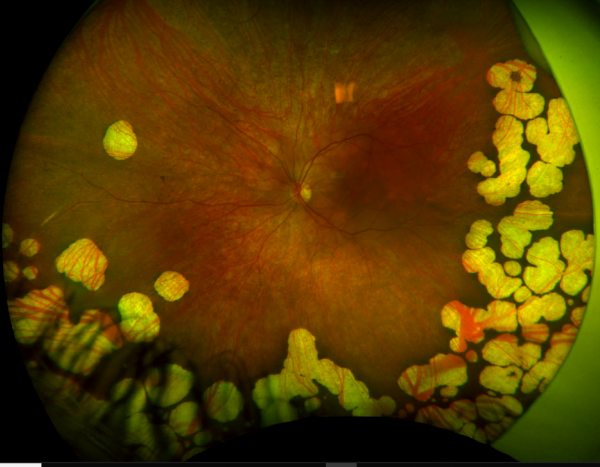
Gyrate Atrophy is due to various mutations in the OAT gene, which is found on chromosome 10q26. Inheritance is autosomal recessive.[4] More than 50 variants have been identified, with missense mutations occurring most frequently. [5][6] These mutations lead to truncation of the enzyme ornithine aminotransferase, causing protein degradation. A subset of patients carry a genetic variant that is favorably responsive to supplementation with co-factor pyridoxine (vitamin B6), which has been shown to partially restore the function of the OAT enzyme. [7] [8] [9][10]
Pathophysiology
The underlying biochemical defect of gyrate atrophy is in ornithine aminotransferase (OAT), a vitamin B6-dependent mitochondrial matrix enzyme. OAT catalyzes the conversion of L-ornithine, a non-proteinaceous amino acid, to proline and glutamic acid, serving in the exchange of molecules between the urea and Kreb’s cycle. OAT plays a critical role in cellular detoxification by disposing of ornithine derived from dietary arginine. [11][12][13][14] A deficiency of OAT thus leads to ornithine accumulation, with levels 10-to 20-fold above normal. [4]
The mechanism by which a deficiency of OAT and the consequent accumulation of ornithine lead to the chorioretinal degeneration is not fully understood. Possibilities include a direct toxic effect of the accumulated ornithine or one of its metabolites. Indeed, animal models have shown that intravitreal injections of ornithine induced the development of degeneration similar to that observed in gyrate atrophy. [15] It has been suggested that the retinal pigment epithelium (RPE) is the site of the initial insult due to its reliance on OAT activity for metabolic functions. RPE injury then leads to a breakdown of the outer blood retinal barrier causing photoreceptor cell exposure to toxic agents from choroidal circulation. [16]
Historically, other theories proposed that damage to the retina might occur secondary to high ornithine concentrations inhibiting creatine biosynthesis, a key agent in cellular energy storage. [17][18] However, creatine supplementation has not been proven to slow retinal disease and its role in pathogenesis remains unclear. [19]
Histology
Histology of atrophic lesions shows complete absence of outer retina, RPE, choriocapillaris, and many of the medium and large choroidal vessels. Directly adjacent to the atrophic areas, there is focal photoreceptor atrophy with underlying hyperplastic RPE. [20]
Diagnosis
Gyrate Atrophy of the choroid and the retina is largely a clinical diagnosis based on history and examination. Elevated levels of systemic ornithine and genetic sequencing confirm the diagnosis.
Symptoms
Patients initially present with myopia and nyctalopia that begins in late childhood. As the disease progresses in the second decade of life, patients report progressive loss of peripheral vision. Visually significant posterior subcapsular cataracts develop early in virtually all patients, frequently by the second decade of life. [21] Vision loss secondary to cystoid macular edema is common. [16]
Exam Findings
Fundus examination early in the disease course shows bilateral patchy, sharply demarcated circular areas of chorioretinal atrophy with hyperpigmented margins in the mid to far periphery (Figure 1). [2] Typically, during the second decade of life, the peripheral atrophic lesions coalesce and spread toward the posterior pole, forming a confluent lesion with a scalloped border at the junction of healthy and diseased retinal pigment epithelium (Figure 2). The remaining healthy RPE in uninvolved areas is hyperpigmented, distinguishing this disease from choroideremia.
The macula and thus central vision is spared until late in the disease course, often preserved into the fourth or fifth decade of life. In a study of 21 patients with long-term follow up and serial fundus angiography, 8 (38%) had macular distribution of the atrophy. [22]
Systemic Features
Non-ocular findings include mild cognitive impairment, delayed language development and speech defects. [23] MR imaging has shown up to 70% have premature atrophic changes in the brain . [24] Other findings include thin, sparse hair and muscular atrophy. [25] There is progressive atrophy of skeletal muscle fibers (specifically type II) both radiographically and histologically, [26][27] although most patients deny muscular symptoms.
Laboratory Tests
Levels of ornithine have been reported to be 10 to 20 times higher than normal in plasma, urine, spinal fluid, and aqueous humor. [4] Gene sequencing analysis is helpful in confirming and identifying the allelic variant. [28]
Other biochemical abnormalities include hypoglutamic acidemia, hypolysenima, hypoglutanemia, and hypoammonemia.[29]
Fundus Photography
Serial fundus photography, especially wide field fundus photography, is useful in monitoring progression of disease. In a study of two related patients with an unrestricted diet and photos taken 24 months apart, younger age and smaller lesion sizes at baseline were associated with a significantly faster rate of chorioretinal degeneration. [30]
Fundus Autofluorescence
Depending on the disease severity, fundus autofluorescence shows either focal or confluent areas of absent autofluorescence, which corresponds with the areas of chorioretinal atrophy seen on fundus exam. These atrophic lesions are commonly surrounded by a sharply demarcated hyperautofluorescent perimeter. The remaining unaffected area, which typically includes the macula, is normal. [16]
Optical Coherence Tomography
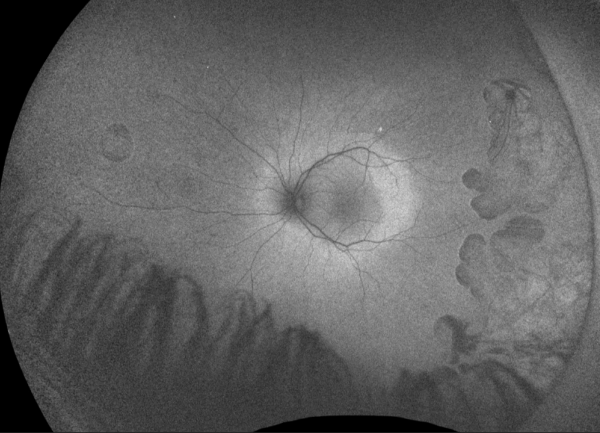
The IS/OS junction is typically preserved in the regions where autofluorescence signal is normal or increased. In the periphery, extensive loss of IS/OS junction, RPE and inner choroid reflectivity, accompanied by significant thinning of both inner and outer retina are often observed concurrently.
Cystoid macular edema is frequent, presumed to be due to impairment of the blood-retinal barrier. The largest case series with SD-OCT imaging showed all 7 (100%) patients were found to have multiple intraretinal cystoid changes and thickening of the fovea. [16] However, it is important to note whether there is petaloid leak at the macula in late phase of fundus fluorescein angiogram. In the presence of vertical tissue bridges, hyporeflective spaces in the retina on SD-OCT, the term foveschisis is used.[1] Macular hole[31] and epiretinal membrane are other possible macular involvement in the gyrate atrophy of the choroid and the retina.
Other findings in advanced disease can include deposits of retinal gliosis, outer retinal tubulation and choroidal neovascularization. [16][32][33]
Electroretinography (ERG)
ERG abnormalities are present at an early stage of the disease, with impaired rod and cone responses, ultimately progressing to a completely extinguished response. [34] Median ERG half-life is 16.0 years (standard combined response) and 10.7 years (flicker response). [35]
Differential Diagnosis
- Choroideremia
- Retinitis Pigmentosa
- Myopic Degeneration
- Cobblestone Degeneration- Spedifically, if the cobblestone degeneration/pavinstone degeneration involves the peripheral and inferior retina extensively it might mimic gyrate atrophy. Examination of the other eye, and family members can give a clue. Pavingstone degeneration usually does not involve posterior to the equator.
- Diffuse Choriocapillaris Atrophy
- Multifocal choroiditis
Management
There is no curative regimen for gyrate atrophy. Lowering the elevated systemic ornithine is presumed to slow disease progression, but the utility of such measures is difficult to prove due to the disease's rarity and slow rate of change.
Refraction and low vision aid should be tried to improve vision and associated quality of life. Examination of the fundus of family members is also important.
Whereas small case series and anecdotal evidence on human subjects suggest that correction of hyperornithinemia seems to be beneficial, animal models have been more definitive in providing histopathologic evidence that early treatment alters the natural history of the disease:
- Low-protein, Arginine-Restricted diet: Arginine is an amino acid derived from dietary protein that serves as the precursor to ornithine (see Figure 3). As a result, adhering to a low-protein diet that restricts arginine consequently lowers systemic levels of ornithine. Animal models with OAT-deficient mice have demonstrated that reducing arginine and thereby ornithine slows progression of ERG and histologic abnormalities when compared to controls.[36] In prospective cohort studies in human subjects, similarly dietary restriction of arginine seems to slow the rate of irreversible vision loss as measured by sequential ERG and visual field examinations,[37][38] more so if started at younger age.[39] Compliance is challenging in light of the highly restrictive nature of the diet and the need for close monitoring by a pediatrician with experience in metabolic disease.[40]
- Vitamin B6 Supplementation: Pyridoxine (vitamin B6) is an essential co-factor to the OAT enzyme. Only a minority of patients have significant reduction in plasma ornithine in response to pharmacologic doses of vitamin B6. [41][42] In a study of 7 patients with gyrate atrophy treated with vitamin B6, three had reduction in ornithine by at least 50%. [43] Only patients with a drop in ornithine levels after vitamin B6 supplementation should be maintained on this regimen. A case report used 400mg of pyridoxine per day.[9] Another study graded the dose of pyridoxal phosphate by none, low (15-18mg per day), and high (500- 750mg/day).[43]
- Creatine Supplementation: High ornithine levels are associated with low systemic creatine levels, a key agent in energy storage. This deficiency has been demonstrated systemically in vivo throughout numerous tissues in patients with gyrate atrophy, particularly muscle and CNS tissue. [44] Historically, patients with retinal findings were treated with high doses of creatine supplementation, with the logic being that systemic creatine deficiency may explain retinal pathology. [45][19] However, long-term benefits to slowing retinal degeneration is unproven, and currently creatine is generally reserved primarily for correction of muscular atrophy.
Cystoid Macular Edema (CME)
There is no consensus regarding treatment of CME. Single case reports have shown improvement with sub-tenon injection of triamcinolone acetonide [46], dexamethasone implantation [47], intravitreal bevacizumab[48], and topical NSAIDs. [49] There is no clear data regarding the utility of systemic carbonic anhydrase inhibitors. One study has shown improvement in two patients with a low-protein, arginine-restricted diet. [50] As mentioned earlier, if is important to differentiate CME from foveoschisis[1] which doe not cause leak of fluorescein on fluorescein angiogram (FA). Typically, CME with peraloid leak on FA responds better to intravitreal/periocular steroid injections, whereas for foveoschisis dozolamide drops and/or acetazolamide tablets are preferred.
Cataract Surgery
Cataracts typically occur by the second decade of life, frequently requiring surgical intervention by the third decade of life. Posterior subcapsular and posterior sutural cataracts are most common. [21] A case series of four eyes reported zonular weakness in all four eyes. [51]
Prognosis
Vision loss is slow and progressive. Considerable variability is observed both in the age at which visual acuities begin to decrease and the age at which visual acuities reach 20/200. Without cataract surgery, the percentage of eyes with acuity 20/200 or worse has been reported as 37% at age 30 and 64% at age 40. In patients with history of cataract surgery, this rate decreased to 20% at age 40. [2] The median visual field half-life of 17.0 years for static perimetry and 11.4 years for kinetic perimetry. [35]
Future Therapeutics
There are no known ongoing clinical trials. As with many of the inherited retinal dystrophies, gene therapy is a promising emerging technology. [52]
References
- ↑ Jump up to: 1.0 1.1 1.2 Tripathy K, Chawla R, Sharma YR, Gogia V. Ultrawide field fluorescein angiogram in a family with gyrate atrophy and foveoschisis. Oman J Ophthalmol. 2016;9(2):104–106. doi:10.4103/0974-620X.184529
- ↑ Jump up to: 2.0 2.1 2.2 Takki K, Milton R. The Natural History of Gyrate Atrophy of the Choroid and Retina. Ophthalmology. 1981 Apr;88(4):292-301.
- ↑ Valle, D., Simell, O. The hyperornithinemias. In: Scriver, C. R.; Beaudet, A. L.; Sly, W. S.; Valle, D. (eds.): The Metabolic and Molecular Bases of Inherited Disease. Vol. II. (8th ed.) New York: McGraw-Hill (pub.) 2001. Pp. 1857-1895.
- ↑ Jump up to: 4.0 4.1 4.2 Simell, O., Takki, K. Raised plasma ornithine and gyrate atrophy of the choroid and retina. Lancet 301: 1031-1033, 1973.
- ↑ Brody, L. C., Mitchell, G. A., Obie, C., Michaud, J., Steel, G., Fontaine, G., Robert, M.-F., Sipila, I., Kaiser-Kupfer, M., Valle, D. Ornithine delta-aminotransferase mutations in gyrate atrophy: allelic heterogeneity and functional consequences. J. Biol. Chem. 267: 3302-3307, 1992.
- ↑ Valle D, Simell O. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C, Beaudet A, Sly W, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1147–1185
- ↑ Montioli R1, Desbats MA2, Grottelli S3, Doimo M2, Bellezza I3, Borri Voltattorni C1, Salviati L4, Cellini B5. Molecular and cellular basis of ornithine δ-aminotransferase deficiency caused by the V332M mutation associated with gyrate atrophy of the choroid and retina. Ophthalmic Genet. 2018 Aug;39(4):512-516.
- ↑ Mashima YG, Weleber RG, Kennaway NG, Inana G. Genotype-phenotype correlation of a pyridoxine-responsive form of gyrate atrophy. Ophthalmic Genet. 1999 Dec;20(4):219-24.
- ↑ Jump up to: 9.0 9.1 Cui X, Jauregui R, Park KS, Tsang SH. Multimodal characterization of a novel mutation causing vitamin B6-responsive gyrate atrophy. Ophthalmic Genet. 2018 Aug;39(4):512-516.
- ↑ Shih VE, Mandell R, Berson EL.Pyridoxine effects on ornithine ketoacid transaminase activity in fibroblasts from carriers of two forms of gyrate atrophy of the choroid and retina. Am J Hum Genet. 1988 Dec;43(6):929-33.
- ↑ ODonnell JJ, Sandman RP, Martin SR (1978) Gyrate atrophy of the retina: inborn error of L-ornithine: 2-oxoacid aminotransferase. Science 200:200–201
- ↑ Volpe P, Sawamura R, Strecker HJ (1969) Control of ornithine delta-transaminase in rat liver and kidney. J Biol Chem 244:719–726
- ↑ Shen BW,Hennig M, Hohenester E et al (1998) Crystal structure of human recombinant ornithine aminotransferase. J Mol Biol 277:81–102
- ↑ Valle D, Walser M, Brusilow SW, Kaiser-Kupfer M (1980) Gyrate atrophy of the choroid and retina: amino acid metabolism and correction of hyperornithinemia with an arginine-deficient diet. J Clin Invest 65:371–378
- ↑ Kuwabara T, Ishikawa Y, Kaiser-Kupfer MI (1981) Experimental model of gyrate atrophy in animals. Ophthalmology 88:331–335
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 Sergouniotis PI, Davison AE, Lenassi E et al (2012) Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology 119:596–605
- ↑ Heinänen K, Näntö-Salonen K, Komu M, Erkintalo M, Heinonen OJ, Pulkki K, Valtonen M, Nikoskelainen E, Alanen A, Simell O. Muscle creatine phosphate in gyrate atrophy of the choroid and retina with hyperornithinaemia--clues to pathogenesis. Eur J Clin Invest. 1999 May;29(5):426-31.
- ↑ Nänto-Salonen K, Komu M, Lundbom N, Heinänen K, Alanen A, Sipilä I, Simell O. Reduced brain creatine in gyrate atrophy of the choroid and retina with hyperornithinemia. Neurology. 1999 Jul 22;53(2):303-7.
- ↑ Jump up to: 19.0 19.1 Sipilä I, Rapola J, Simell O, Vannas A. Supplementary Creatine as a Treatment for Gyrate Atrophy of the Choroid and Retina. N Engl J Med 1981; 304:867-870
- ↑ Wilson DJ, Weleber RG, Green WR (1991) Ocular clinicopathologic study of gyrate atrophy. AmJ Ophthalmol 111:24–33
- ↑ Jump up to: 21.0 21.1 Kaiser-Kupfer M, Kuwabara , Uga S, Takki K, Valle D. Cataracts in gyrate atrophy: clinical and morphologic studies. Invest. Ophthal. Vis. Sci. 24: 432-436, 1983
- ↑ Kaarina Vannas‐Sulonen, Progression of gyrate atrophy of the choroid and retina. A long‐term follow‐up by fluorescein angiography. Acta Ophthalmologica. Volume 65, issue 1. Feb 1987: 101-109
- ↑ Stoppoloni G, Prisco F, Santinelli R, Tolone C. Hyperornithinemia and gyrate atrophy of choroid and retina. Report of a case. Helv Paediatr Acta. 1978;33(4-5):429-33.
- ↑ Valtonen, M., Nanto-Salonen, K., Jaaskelainen, S., Heinanen, K., Alanen, A., Heinonen, O. J., Lundbom, N., Erkintalo, M., Simell, O. Central nervous system involvement in gyrate atrophy of the choroid and retina with hyperornithinaemia. J. Inherit. Metab. Dis. 22: 855-866, 1999
- ↑ Lahbil D, Hamdani M, D’khissy M et al (2000) Gyrate atrophy of the choroid and retina: a case report. J Fr Ophthalmol 23:788–793
- ↑ Sipila, I., Simell, O., Rapola, J., Sainio, K., Tuuteri, L. Gyrate atrophy of the choroid and retina with hyperornithinemia: tubular aggregates and type 2 fiber atrophy in muscle. Neurology 29: 996-1005, 1979.
- ↑ Valtonen, M., Nanto-Salonen, K., Heinanen, K., Alanen, A., Kalimo, H., Simell, O. Skeletal muscle of patients with gyrate atrophy of the choroid and retina and hyperornithinaemia in ultralow-field magnetic resonance imaging and computed tomography. J. Inherit. Metab. Dis. 19: 729-734, 1996.
- ↑ https://www.ncbi.nlm.nih.gov/gtr/all/tests/?term=C0599035[DISCUI]&filter=method:2_7;testtype:clinical
- ↑ Valle D, Walser M, Brusilow S, Kaiser-Kupfer MI, Takki K. Gyrate atrophy of the choroid and retina. Biochemical considerations and experience with an arginine-restricted diet. Ophthalmology. 1981;88(4):325–330. doi:10.1016/s0161-6420(81)35028-3
- ↑ Salcedo-Villanueva G, Paciuc-Beja M, Villanueva-Mendoza C, Harasawa M, Smith JM, Velez-Montoya R, Olson JL, Oliver SC, Mandava N, Quiroz-Mercado H. Progression of gyrate atrophy measured with ultra-widefield imaging. Int Ophthalmol (2016) 36: 111.
- ↑ Tripathy K, Sharma YR, Chawla R, Jain S, Behera A. Ultra-wide Field Imaging of an Operated Macular Hole in Gyrate Atrophy. J Ophthalmic Vis Res. 2016;11(3):336–337. doi:10.4103/2008-322X.188404
- ↑ Chatziralli I, Theodossiadis G, Emfietzoglou I, Theodossiadis P. Intravitreal ranibizumab for choroidal neovascularization secondary to gyrate atrophy in a young patient: a multimodal imaging analysis. Eur J Ophthalmol. 2015 Oct 21;25(6):e119-22.
- ↑ Inanc M, Tekin K, Teke MY. Bilateral choroidal neovascularization associated with gyrate atrophy managed with intravitreal bevacizumab. Int Ophthalmol. 2018 Jun;38(3):1351-1355.
- ↑ Raitta, C., S. Carlson, and K. Vannas-Sulonen.Gyrate atrophy of the choroid and retina: ERG of the neural retina and the pigment epithelium. Br. J. Ophthalmol. 1990; 74: 363-367
- ↑ Jump up to: 35.0 35.1 Caruso, R. C., Nussenblatt, R. B., Csaky, K. G., Valle, D., Kaiser-Kupfer, M. I. Assessment of visual function in patients with gyrate atrophy who are considered candidates for gene replacement. Arch. Ophthal. 119: 667-669, 2001.
- ↑ Wang T, Steel G, Milam AH, Valle D. Correction of ornithine accumulation prevents retinal degeneration in a mouse model of gyrate atrophy of the choroid and retina. Proc Natl Acad Sci USA. 2000 Feb 1;97(3):1224-9.
- ↑ Kaiser-Kupfer MI, Caruso RC, Valle D, Reed GF (2004). Use of an arginine-restricted diet to slow progression of visual loss in patients with gyrate atrophy. Arch Ophthalmol122:982–984
- ↑ Wang T, Steel G, Milam AH, Valle D. Correction of ornithine accumulation prevents retinal degeneration in a mouse model of gyrate atrophy of the choroid and retina. Proc Natl Acad Sci U S A. 2000 Feb 1;97(3):1224-9.
- ↑ Kaiser-Kupfer MI, Caruso RC, Valle D. Gyrate atrophy of the choroid and retina: further experience with long-term reduction of ornithine levels in children. Arch Ophthalmol. 2002 Feb;120(2):146-53.
- ↑ Wang T, Steel G, Milam AH, Valle D (2000) Correction of ornithine accumulation prevents retinal degeneration in a mouse model of gyrate atrophy of the choroid and retina. Proc Natl Acad Sci USA 97:1224–1229
- ↑ Michaud J, Thompson GN, Brody LC et al. Pyridoxine-responsive gyrate atrophy of the choroid and retina: clinical and biochemical correlates of the mutation A226V. Am J Hum Genet. 1995; 56(3): 616-22.
- ↑ S. Hayasaka, T. Saito, H. Nakajima, O. Takahashi, K. Mizuno, and K. Tada. Clinical trials of vitamin B6 and proline supplementation for gyrate atrophy of the choroid and retina. Br J Ophthalmol. 1985 Apr; 69(4): 283–290.
- ↑ Jump up to: 43.0 43.1 Weleber RG, Kennaway NG. Clinical trial of vitamin B6 for gyrate atrophy of the choroid and retina. Ophthalmology. 1981 Apr;88(4):316-24.
- ↑ Nänto-Salonen K1, Komu M, Lundbom N, Heinänen K, Alanen A, Sipilä I, Simell O. Reduced brain creatine in gyrate atrophy of the choroid and retina with hyperornithinemia. Neurology. 1999 Jul 22;53(2):303-7.
- ↑ Valtonen, M., Nanto-Salonen, K., Jaaskelainen, S., Heinanen, K., Alanen, A., Heinonen, O. J., Lundbom, N., Erkintalo, M., Simell, O. Central nervous system involvement in gyrate atrophy of the choroid and retina with hyperornithinaemia. J. Inherit. Metab. Dis. 22: 855-866, 1999
- ↑ Alparslan Ş, Fatih MT, Muhammed Ş, Adnan Y. Cystoid macular edema secondary to gyrate atrophy in a child treated with sub-tenon injection of triamcinolone acetonide. Rom J Ophthalmol. 2018 Jul-Sep;62(3):246-249.
- ↑ Abdelmassih Y, El-Khoury S, Cherfan CG. Dexamethasone implant for the treatment of gyrate atrophy associated macular edema. J Fr Ophtalmol. 2019 Jan;42(1):e1-e4.
- ↑ Elnahry AG, Hassan FK, Abdel-Kader AA. Bevacizumab for the treatment of intraretinal cystic spaces in a patient with gyrate atrophy of the choroid and retina. Ophthalmic Genet. 2018 Dec;39(6):759-762.
- ↑ Piozzi E, Alessi S, Santambrogio S, Cillino G, Mazza M, Iggui A, Cillino S. Carbonic Anhydrase Inhibitor with Topical NSAID Therapy to Manage Cystoid Macular Edema in a Case of Gyrate Atrophy. Eur J Ophthalmol. 2017 Nov 8;27(6):e179-e183.
- ↑ Casalino, Giuseppe, Luisa Pierro, Maria Pia Manitto, Michel Michaelides, and Francesco Bandello. Resolution of Cystoid Macular Edema following Arginine-restricted Diet and Vitamin B6 Supplementation in a Case of Gyrate Atrophy. Journal of AAPOS. 22.4: 321-23.
- ↑ Chao J, Rao P, Hart J. Zonular weakness in patients with gyrate atrophy of the choroid and retina. JCRS Online Case Reports. October 2017. 5(4): 69-72.
- ↑ DeBenedictis, M, Rachitskaya A. First Gene Therapy FDA-Approved for an Inherited Retinal Disease: The approval has stimulated research into gene therapies for other IRDs. Retina Today. http://retinatoday.com/2018/04/first-gene-therapy-fda-approved-for-an-inherited-retinal-disease. Accessed 03.31.19.