Enhanced S-Cone Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease
Enhanced S Cone syndrome (ESCS) is a relatively newly described and very rare inherited progressive retinal degeneration that can cause severe vision loss. The characteristic electroretinogram (ERG) findings were first described in 1990 and are important in the diagnosis of this genetic disease.[1]
Etiology
ESCS is a progressive autosomal recessive retinal degeneration caused by a mutation in nuclear receptor subfamily 2, group E, member 3 (NR2E3) gene and rarely in the NRL gene.
General Pathology
The mutation of the NR2E3 receptor results in a gain of function in S cones. The S cone is one of three types of retinal cones and is the least common cone in the normal human retina. During embryology, the malfunctioning NR2E3 transcription factor is hypothesized to result in a differentiation error resulting in too many S cones being produced and no rods.[2]
In rare cases, mutations in the NRL gene, which acts upstream of the NR2E3 transcription factor, can lead to ESCS.[3]
Diagnosis
Electroretinographic findings are critical in the diagnosis of ESCS showing no rod response; both scotopic and photopic ERG wave forms are similar. [4]
History
ESCS typically presents in childhood with nyctalopia. Other presenting symptoms include refractive accommodative esotropia, nystagmus, decreased visual acuity, hemeralopia, and incidental retinal lesions on examination.[5]
Physical examination
There is a broad range of disease severity. Visual acuity can range from normal to profoundly impaired, with approximately 30% of patients having a visual acuity of 20/100 or worse.[6] Color vision is typically diminished with the preservation of the tritan axis and incraesed sensitivity to blue light. The night blindness exists from birth.
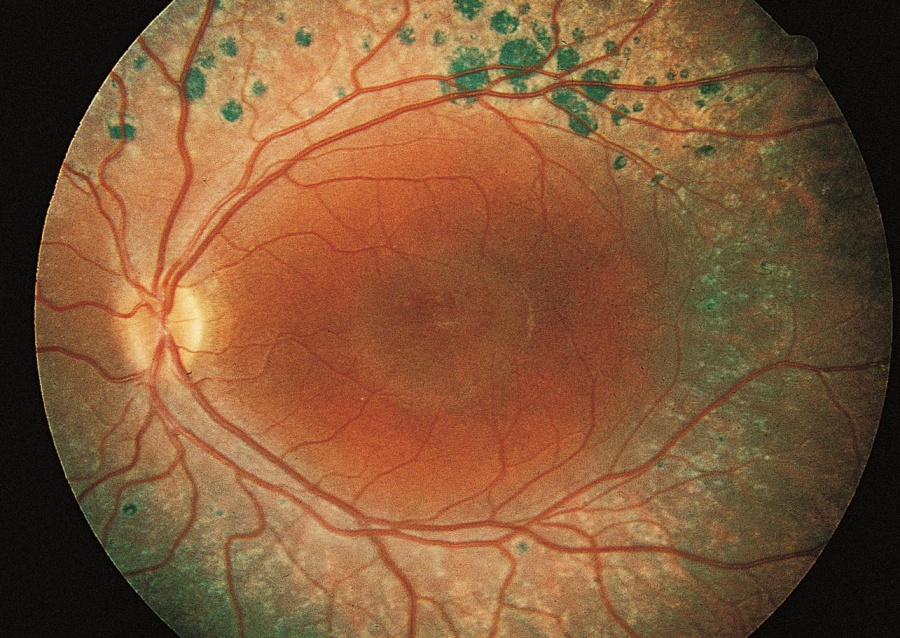
The characteristic findings on exam are nummular pigmentary changes along the vascular arcades (Figure 1), found in about 85% of patients.[6] Approximately 50% of patients will have subretinal yellow-white lesions in the posterior pole, and these can then progress to the more classic pigmented lesions.[5][6] Other posterior pole findings include circumferential submacular fibrosis and torpedo-like atrophic lesions.[7]
There are no systemic associations and patients can expect a normal life expectancy.
Clinical diagnosis
Diagnosis is confirmed by pathognomonic findings on ERG and can be confirmed by genetic testing.
Diagnostic procedures
ERG is critical for diagnosis and shows an absent rod response on low-intensity dark-adapted stimulus and a similar wave from to single white light flashes in both scotopic and photopic conditions. The 30 Hz flicker response is of low amplitude and delayed. To elicit an S cone response, a blue wavelength is given on an orange background (suppressing red and green cones), resulting in an S cone response that is greater than normal and is the reason for the name of this disease.[4]
Optical coherence tomography (OCT) can demonstrate foveal schisis and the majority of patients with severe vision loss have advanced schisis.[6] These schisis cavities can involute into areas of advanced atrophy. The torpedo-like lesions are associated with RPE atrophy and subretinal hyperreflective deposits.[7] Subretinal fibrosis due to choroidal neovascularization can be seen in some advanced cases.[7]
Fundus autofluorescence reveals decreased autofluorescence in the peripheral retina with relatively increased autofluorescence in the macula. [4] The yellow-white lesions are hyperautofluorescent.[6]
Laboratory test
Genetic testing for the NR2E3 gene can be sent to confirm the diagnosis and can be helpful in ambiguous cases.
Differential diagnosis
Nummular pigmentary deposits, the most common clinical exam finding in patients with ESCS, can be seen in other retinal dystrophies, including Bardet-Biedl syndrome, CRB1-associated early-onset severe retinal dystrophy, retinitis pigmentosa with preserved para-arteriolar RPE, and thioridazine retinopathy.[6]
The differential for nyctalopia in children includes inherited retinal degenerations (congenital stationary night blindness, retinitis pigmentosa, fundus albipunctatus, Oguchi disease, choroideremia), optic nerve pathology (glaucoma and other causes), vitamin A deficiency, autoimmune retinopathy, and cataracts.
Goldman-Favre syndrome is caused by the same mutation as in ESCS and is a peripheral retinal degeneration with foveal schisis. It is considered to be on the spectrum of ESCS disease.
Management
Medical therapy
Macular schisis can be treated with oral or topical carbonic anhydrase inhibitors, although efficacy is variable.[6][8][9][10][11]
Choroidal neovascularization can be treated with anti-vascular endothelial growth factor agents and has been used in children as young as 5 years.[12][13]
Medical follow up
Children should be monitored for refractive error and macular status. OCT can be performed to monitor for schisis and choroidal neovascularization.
Prognosis
Enhanced S cone syndrome is a slowly progressive retinal degeneration that has significant clinical variability. Some patients have extensive degeneration and vision decline while others show little progression and maintain excellent visual acuity.[14]
Additional resources
Examples of fundus images and ancillary testing can be seen in these open access articles:
Expanded Clinical Spectrum of Enhanced S Cone Syndrome
Enhanced S Cone Syndrome in Children
Phenotypic Variation in Enhance S Cone Syndrome
References
- ↑ Jacobson SG, Marmor MF, Kemp CM, Knighton RW. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci. 1990;31(5):827-838.
- ↑ Haider NB, Mollema N, Gaule M, et al. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res. 2009;89(3):365-372. doi:10.1016/j.exer.2009.04.006
- ↑ Littink KW, Stappers PTY, Riemslag FCC, et al. Autosomal Recessive NRL Mutations in Patients with Enhanced S-Cone Syndrome [published correction appears in Genes (Basel). 2018 Mar 07;9(3):]. Genes (Basel). 2018;9(2):68. Published 2018 Jan 30. doi:10.3390/genes9020068
- ↑ 4.0 4.1 4.2 Tsang SH, Sharma T. Enhanced S-Cone Syndrome (Goldmann-Favre Syndrome). Adv Exp Med Biol. 2018;1085:153-156. doi: 10.1007/978-3-319-95046-4_28. PMID: 30578501.
- ↑ 5.0 5.1 Khan, A. O., Aldahmesh, M., & Meyer, B. (2009). The enhanced S-cone syndrome in children. BMJ case reports, 2009, bcr10.2008.1163. https://doi.org/10.1136/bcr.10.2008.1163
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 de Carvalho ER, Robson AG, Arno G, Boon C, Webster AA, Michaelides M. Enhanced S-cone syndrome: spectrum of clinical, imaging, electrophysiological and genetic findings in a retrospective case series of 56 patients [published online ahead of print, 2020 Jul 14]. Ophthalmol Retina. 2020;S2468-6530(20)30286-4. doi:10.1016/j.oret.2020.07.008
- ↑ 7.0 7.1 7.2 Yzer S, Barbazetto I, Allikmets R, et al. Expanded clinical spectrum of enhanced S-cone syndrome. JAMA Ophthalmol. 2013;131(10):1324-1330. doi:10.1001/jamaophthalmol.2013.4349
- ↑ Bušić M, Bjeloš M, Bosnar D, Ramić S, Bušić I. Cystoid macular lesions are resistant to topical dorzolamide treatment in enhanced S-cone syndrome child. Doc Ophthalmol. 2016;132(1):67-73. doi:10.1007/s10633-016-9527-0
- ↑ Genead MA, Fishman GA, McAnany JJ. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol. 2010;121(3):231-240. doi:10.1007/s10633-010-9247-9
- ↑ Hajali M, Fishman GA. Dorzolamide use in the management of macular cysts in a patient with enhanced s-cone syndrome. Retin Cases Brief Rep. 2009;3(2):121-124. doi:10.1097/ICB.0b013e31818faa21
- ↑ Iannaccone A, Fung KH, Eyestone ME, Stone EM. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol. 2009;147(2):307-312.e2. doi:10.1016/j.ajo.2008.08.003
- ↑ Broadhead GK, Grigg JR, McCluskey P, Korsakova M, Chang AA. Bevacizumab for choroidal neovascularisation in enhanced S-cone syndrome. Doc Ophthalmol. 2016;133(2):139-143. doi:10.1007/s10633-016-9555-9
- ↑ Bertoli F, Pignatto S, Rizzetto F, Lanzetta P. A 5-Year-Old Case of Choroidal Neovascularization in Enhanced S-Cone Syndrome Treated with Ranibizumab. Case Rep Ophthalmol. 2018;9(3):510-515. Published 2018 Dec 21. doi:10.1159/000495743
- ↑ Marmor MF. An Examination of the Propositus of Enhanced S-Cone Syndrome 30 Years After Diagnosis [published online ahead of print, 2020 Jul 23]. JAMA Ophthalmol. 2020;10.1001/jamaophthalmol.2020.2556. doi:10.1001/jamaophthalmol.2020.2556