Congenital Stationary Night Blindness (CSNB)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Congenital Stationary Night Blindness (CSNB) is recognized by the code H53.63 as per the International Classification of Diseases Version 10 (ICD-10) nomenclature.
Disease
CSNB is a heterogenous collection of rare genetic diseases affecting photoreceptors, the retinal pigment epithelium (RPE), or bipolar cells. Generally, affected individuals exhibit non-progressive dark or dim-light visual difficulties (nyctalopia) starting from birth.[1]
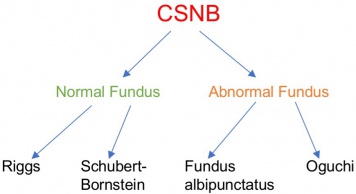
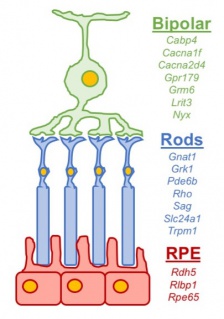
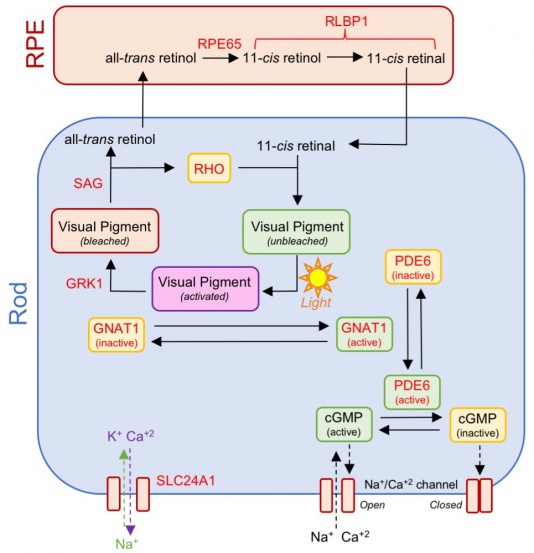
Classically CSNB has been categorized into those with normal appearing fundi and those with retinal changes (Figure 1). CNSB without fundus abnormalities can be subdivided into two categories based on electroretinogram (ERG) findings: 1) Riggs-type and 2) Schubert-Bornstein which can be further subdivided into complete (cCSNB) and incomplete (iCSNB) subtypes .[2][3][4] cCSNB is characterized by a defect that localizes to the ON bipolar cells, leading to an dysfunction in transmission through the bipolar cells which is evidenced by a lack of the b-wave on scotopic ERG. In iCSNB, the defect is localized to the photoreceptor synapse, leading to altered signaling to both ON and OFF bipolar cells which is illustrated by a diminished but recordable rod ERG response.[4] Fundus albipunctatus and Oguchi disease are two entities within CSNB that are associated with fundus findings.[1] The specific clinical and ERG findings (see Clinical Diagnosis) in each subtype can be specifically explained by the gene mutation and its relation to the phototransduction cascade (Figure 2)(Figure 3).
Etiology
Current research has implicated numerous genetic mutations primarily affecting 17 different genes involved in phototransduction and post-phototransduction transmission (Table 1). The four subtypes of CSNB have different genetics defects which correspond to a specific ERG dysfunction. The known mutations associated with complete Schubert-Bornschein include an X-linked mutation in the NYX gene and autosomal recessive mutations in the GRM6, TRPM1, GPR179 or LRIT3 genes which are expressed on the dendrites of bipolar cells. Most cases of incomplete Schubert-Bornschein are secondary to an X linked mutation of CACNA1F or CABP4.[5][6] Known mutations for Riggs-type CSNB include autosomal dominant mutations in GNAT1 and PDE6B which are involved in rod phototransduction.[7] Recently an autosomal recessive mutation in SCL24A1 has been found to cause Riggs-type CSNB.[8] Fundus albipunctatus is caused by an autosomal recessive mutation of the RDH5 gene which is involved in retinoid recycling.[9] Lastly Oguchi disease is associated an autosomal recessive mutation in either the GRK1 or SAG gene.[10][11]
| Mutation | Inheritance | Encoded Protein Function | Electroretinogram (ERG) Findings |
|---|---|---|---|
| Cabp4 | AR | Calcium binding protein within bipolar cells |
|
| Cacna1f | X | Subunit of a calcium voltage-gated channel within bipolar cells |
|
| Cacna2d4 | AR | Subunit of a calcium voltage-gated channel within bipolar cells |
|
| Gnat1 | AD | Subunit of transducin involved in rod phototransduction |
|
| Gpr179 | AR | Glutamate receptor on surface of bipolar cells involved in signal transmission from rods |
|
| Grk1 | AR | G-protein coupled receptor kinase within rods responsible for phosphorylating activated rhodopsin to deactivate the phototransduction cascade |
|
| Grm6 | AR | Glutamate receptor on surface of bipolar cells involved in signal transmission from rods |
|
| Lrit3 | AR | Regulatory protein required for proper localization of ion channels encoded by Trpm1 in bipolar cells |
|
| Nyx | X | Nyctalopin protein (function unknown) within bipolar cells involved in signal transmission from rods |
|
| Pde6b | AD | Subunit of phosphodiesterase protein involved in rod phototransduction |
|
| Rdh5 | AR | Retinol dehydrogenase converts 11-cis retinol to 11-cis retinal within the RPE to promote visual cycle function |
|
| Rho | AD | G-protein coupled receptor involved in rod phototransduction |
|
| Rlbp1 | AR | Binding protein for stabilizing 11-cis retinal and 11-cis retinol within the RPE to promote visual cycle function |
|
| Rpe65 | AR | Involved in utilizing 11-cis retinol within the RPE and cones to promote visual cycle function (exact function unknown) |
|
| Sag | AR | Arrestin protein involved in desensitizing the phototransduction cascade within rods |
|
| Slc24a1 | AR | Subunit of a potassium-dependent sodium/calcium channel exchanger involved in rod phototransduction |
|
| Trpm1 | AR | Ion channel within bipolar cells involved in signal transmission from rods |
|
General Pathology
CSNB is a retinal disease that primary affects signaling processing within rod photoreceptors, retinoid recycling in the RPE, and signal transmission via bipolar cells (Figure 2). Seventeen genes with more than 360 mutations and 670 alleles have been found to be associated with CSNB (Table 1, Figure 3).[1]
Primary Prevention
There is currently no preventative measures for this disease.
Diagnosis
History
A detailed personal and family history for night blindness and/or decreased vision should be elicited. Classically it was thought that patients with CSNB present with nyctalopia from birth though recent evidence indicates that not all patients are aware of their night vision dysfunction. [5] [12] In a review of children with iCSNB only 54% of patients presented with nyctalopia, and thus it is important for clinicians to not "rule out" CSNB from the differential if there is no complaint of nyctalopia.[5]
Physical Examination
Patients should undergo a full ophthalmic examination, including a dilated fundus exam to evaluate for congenital night blindness with fundus abnormalities. Visual acuity is typically reduced with a median of 20/40 in cCSNB and 20/60 in iCSNB.[5] Additionally, formal color vision testing should be performed as a small minority of patients with cCSNB will have dysfunction with color vision.[1] In Riggs-Type and Schubert Bornschein CSNB the fundus is normal other than myopic changes which are commonly found.[13]
Signs & Symptoms
Patients with CSNB may complain of poor night or dim-light vision. These symptoms are often subjective and may not be appreciated by those who live in well-lit urban areas. Photophobia is a common complaint especially in bright light conditions. Patients can also present with myopia, strabismus, and nystagmus. Eye-movement recordings in CSNB patients reveal a predominantly disconjugate pendular nystagmus of small amplitude, high frequency, and oblique direction.[1]
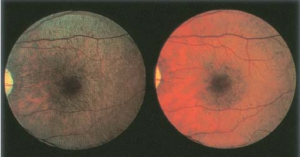
Fundus albipunctatus and Oguchi disease are two entities within CSNB that are associated with fundus findings. Patients with fundus albipunctatus demonstrate scattered yellow-white dots in the posterior pole (sparing the macula) that extend to the mid-periphery.[1] These dots may disappear over time though they are typically stable.[14] They are presumed to contain 11-cis retinal precursors (retinoids) and exist from the RPE/Bruch membrane complex to outer nuclear layer.[15] Those with Oguchi disease demonstrate the Mizuo-Nakamura phenomenonin which the fundus is unremarkable in the dark-adapted state but has a yellow iridescent (golden) sheen after light exposure (Figure 4). The mechanism underlying this process is currently not well understood.[1]
Clinical Diagnosis
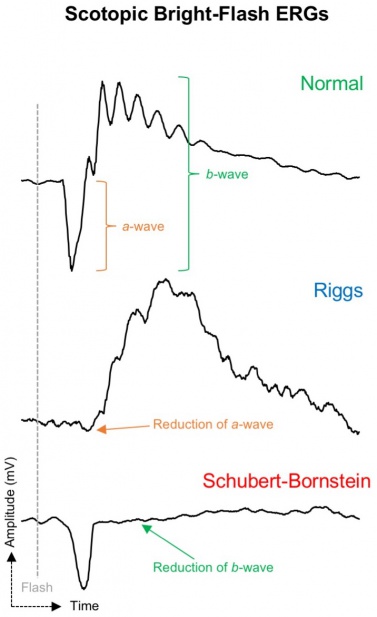
Electroretinography (ERG) is the most valuable ancillary test in distinguishing subtypes of CSNB. Riggs-Type CSNB and Schubert-Bornstein CSNB have pathognomonic full-field ERG tracings which are used to distinguish the two entities (Figure 5).
The Riggs subtype is associated with photoreceptor dysfunction which presents with selective loss of rod function. The dim flash scotopic ERG is flat whereas the strong flash scotopic ERG shows a decreased a-wave and b-wave amplitude which is in contradistinction to the Schubert-Bornstein which demonstrates a normal a-wave. Photopic ERG remains normal, indicating preserved cone function.[1]
The Schubert-Bornstein subtype is associated with bipolar cell dysfunction. The ERG reflects dysfunction in signaling between photoreceptors and bipolar cells or a post-phototransduction transmission defect. The most common pattern observed is an electronegative scotopic ERG, characterized by a normal a-wave (as phototransduction in the rod photoreceptors is still functional) but a reduced b-wave (due to bipolar transmission dysfunction).[1]
The complete form of Schubert-Bornstain is associated with ON bipolar pathway dysfunction. Photopic ERG often exhibits a normal a-wave but with a broadened trough and a sharply increasing b-wave with loss of oscillatory potentials. A long response stimulus can be used to confirm the diagnosis: the ON pathway shows the characteristic negative ERG while the OFF pathway is normal.[1] The incomplete form of CSNB is associated with ON and OFF pathway dysfunction. The scotopic dim-flash ERG signal is present, but the amplitude of the a-wave is diminished, while the bright-flash ERG shows an electronegative waveform. The photopic response is more severely affected compared to the complete form: the flicker ERG signal is delayed and often displays a bifid peak. The difference is due to residual rod function.[4]
Fundus albipunctatus has a full-field ERG tracing similar to those with Riggs-type CSNB though there is often some detectable a-wave with dim flash scotopic ERG.[13] Interestingly with prolonged dark adaptation the scotopic ERGs often normalize.[16] Oguchi disease also has a full-field ERG tracing similar to Riggs-type CSNB though with improved bright flash rod responses to prolonged dark adaptation (1-2 hours) similar to Fundus Albipunctatus.[1][17]
Diagnostic procedures
ERG plays a critical role in the diagnosis of CSNB. As previously described the ERG is crucial to distinguish the four subtypes of CSNB and also assists in distinguishing between cCSNB and iCSNB.
Optical coherence tomography (OCT) can be helpful in evaluating fundus albipunctatus and Oguchi disease. In Fundus albipunctatus there are hyperreflective deposits in the RPE that extend up to the outer nuclear layer which correspond to the visualized dots on fundus examination.[15][18] OCT studies in Oguchi disease hypothesize that the sheen is due to an accumulation of material (presumably rhodopsin) in the shortened rod outer segments.[19]
Fundus autofluorescence typically demonstrates a decreased background autofluoresence which is consistent with a dysfunctional retinoid cycle.[1]
Laboratory test
Once the specific subtype of CSNB has been elucidated based on clinical and ERG findings, selective gene testing can be procured.
Differential diagnosis
The differential diagnosis for CSNB includes retinitis pigmentosa, progressive rod-cone dystrophy, acquired night blindness (typically Vitamin A deficiency), and retinitis punctata albscens (mimics fundus albipunctatus).
Compared to CSNB, which is nonprogressive, retinitis punctata albescence is progressive and leads to increasing symptoms and gradual deterioration of ERG and visual fields.
Misdiagnosis is very common and patients are typically diagnosed with strabismus, myopia or congenital motor nystagmus before CSNB is eventually diagnosed.[12]
Management
There are currently no treatments for CSNB. However, a small nonrandomized prospective study of seven patients with fundus albipunctatus (defect in RDH5 gene) treated with high dose oral 9-cis-beta-carotene demonstrated improvement in visual field and ERG testing.[20] Photoreceptor replacement by transplantation and gene therapy are modalities under investigation that may be paradigm shifting in managing CSNB.
Prognosis
Generally, the clinical course of patients with CSNB does not change over time. The longest follow up documented in the literature is a patient who was followed for 38 years. Further accumulation of clinical date is needed to establish prognostic factors for CSNB.[21]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Baldwin, A. N., Robson, A. G., Moore, A. T., & Duncan, J. L. (2018). Ryans retina (pp. 1006-1017) (A. P. Schachat, Ed.). Edinburgh: Elsevier.
- ↑ Schubert G, and Bornschein H: Analysis of the human electroretinogram. Ophthalmologica 1952; 123: pp. 396-413
- ↑ Riggs LA: Electroretinography in cases of night blindness. Am J Ophthalmol 1954; 38: pp. 70-78
- ↑ Jump up to: 4.0 4.1 4.2 Miyake Y, Yagasaki K, Horiguchi M, et al: Congenital stationary night blindness with negative electroretinogram: a new classification. Arch Ophthalmol 1986; 104: pp. 1013-1020
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Bijveld MM, Florijn RJ, Bergen AA, et al: Genotype and phenotype of 101 Dutch patients with congenital stationary night blindness. Ophthalmology 2013; 120: pp. 2072-2081
- ↑ Zeitz C, Robson AG, and Audo I: Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res 2015; 45: pp. 58-110
- ↑ Dryja TP: Molecular genetics of Oguchi disease, fundus albipunctatus, and other forms of stationary night blindness: LVII Edward Jackson Memorial Lecture. Am J Ophthalmol 2000; 130: pp. 547-563
- ↑ Riazuddin SA, Shahzadi A, Zeitz C, et al: A mutation in SLC24A1 implicated in autosomal-recessive congenital stationary night blindness. Am J Hum Genet 2010; 87: pp. 523-531
- ↑ Yamamoto H, Simon A, Eriksson U, et al: Mutations in the gene encoding 11- . Nat Genet 1999; 22: pp. 188-191
- ↑ Yamamoto S, Sippel KC, Berson EL, et al: Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nat Genet 1997; 15: pp. 175-178
- ↑ Fuchs S, Nakazawa M, Maw M, et al: A homozygous 1-base pair deletion in the arrestin gene is a frequent cause of Oguchi disease in Japanese. Nat Genet 1995; 10: pp. 360-362
- ↑ Jump up to: 12.0 12.1 Miraldi utz V, Pfeifer W, Longmuir SQ, Olson RJ, Wang K, Drack AV. Presentation of TRPM1-Associated Congenital Stationary Night Blindness in Children. JAMA Ophthalmol. 2018;136(4):389-398.
- ↑ Jump up to: 13.0 13.1 Sergouniotis PI, Robson AG, Li Z, et al: A phenotypic study of congenital stationary night blindness (CSNB) associated with mutations in the GRM6 gene. Acta Ophthalmol 2011; 90: pp. e192-7
- ↑ Sekiya K, Nakazawa M, Ohguro H, et al: Long-term fundus changes due to fundus albipunctatus associated with mutations in the RDH5 gene. Arch Ophthalmol 2003; 121: pp. 1057-1059
- ↑ Jump up to: 15.0 15.1 Querques G, Carrillo P, Querques L, et al: High-definition optical coherence tomographic visualization of photoreceptor layer and retinal flecks in fundus albipunctatus associated with cone dystrophy. Arch Ophthalmol 2009; 127: pp. 703-706
- ↑ Cideciyan AV, Haeseleer F, Fariss RN, et al: Rod and cone visual cycle consequences of a null mutation in the 11- . Vis Neurosci 2000; 17: pp. 667-678
- ↑ Carr RE, and Gouras P: Oguchi's disease. Arch Ophthalmol 1965; 73: pp. 646-656
- ↑ Genead MA, Fishman GA, and Lindeman M: Spectral-domain optical coherence tomography and fundus autofluorescence characteristics in patients with fundus albipunctatus and retinitis punctata albescens. Ophthalmic Genet 2010; 31: pp. 66-72
- ↑ Hashimoto H, and Kishi S: Shortening of the rod outer segment in Oguchi disease. Graefes Arch Clin Exp Ophthalmol 2009; 247: pp. 1561-1563
- ↑ Rotenstreich Y, Harats D, Shaish A, et al: Treatment of a retinal dystrophy, fundus albipunctatus, with oral 9-cis-{beta}-carotene. Br J Ophthalmol 2010; 94: pp. 616-621
- ↑ Kurata K, Hosono K, Hotta Y. Long-Term Clinical Course in a Patient with Complete Congenital Stationary Night Blindness. Case Rep Ophthalmol. 2017;8(1):237-244.