Doyne Honeycomb Retinal Dystrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Doyne Honeycomb Retinal Dystrophy, also known as Malattia Leventinese or Familial Dominant Drusen, all refer to the same genetic inherited retinal dystrophy characterized by an autosomal dominant mutation in the EFEMP1 gene in which patients develop early onset macular and peripapillary drusen that are often oriented radially, initially described as a "Honeycomb" pattern by Doyne in 1899.
Disease Entity
Doyne Honeycomb Retinal Dystrophy (DHRD) or Malattia Leventinese (MLVT) or Dominant Drusen can be coded under the header H35.5 for Hereditary Retinal Dystrophy.
- H35.50 - Unspecified hereditary retinal dystrophy
- H35.51 - Vitreoretinal dystrophy
- H35.52 - Pigmentary retinal dystrophy
- H35.53 - Other dystrophies primarily involving the sensory retina
- H35.54 - Dystrophies primarily involving the retinal pigment epithelium
Disease and History
Doyne Honeycomb Retinal Dystrophy (DHRD) was first described phenotypically by Doyne in 1899 in four sisters in England[1]. He found that each had an early onset retinal dystrophy with closely grouped white lesions in the macula and disc area which he termed "Honeycomb" pattern. Vogt in 1925 then described a similar phenotypic picture in a cluster of likely related individuals in the Leventine Valley of Switzerland, thus spawning the name Malattia Leventinese (MLVT) [2]. Ophthalmologists did suspect for a period of time that the two separately described entities likely referenced the same disease given their similarities, and in 1996 both DHRD and MLVT were mapped to abnormalities in chromosome 2, first by Heon in patients with MLVT[3], then by Gregory shortly after for patients with DHRD[4]. Even in light of this discovery in 1996, it was still thought that the two diseases may represent separate entities.
However in 1999, Stone et al.[5] identified a single mutation in the EFEMP1 gene on chromosome 2 in both families with DHRD and MLVT, confirming that the two represented slight phenotypic variances of the same disease, and thus we now consider them to be the same clinical entity.
Risk Factors
Since Doyne Honeycomb Retinal Dystrophy results from an autosomal dominant mutation in the EFEMP1 gene, having an affected family member is the only identifiable risk factor
Pathophysiology and Genetics
Doyne Honeycomb Retinal Dystrophy is characterized by an autosomal dominant mutation in the EFEMP1 gene, specifically a single missense Arg345Trp (R345W) mutation in exon 10.
EFEMP1 stands for EGF-containing fibulin-like extracellular matrix protein 1, and the gene coding for it lies on chromosome 2p16.
Normally, EFEMP1 is widely expressed in the extracellular matrix, however, its exact function is unknown.
Fu et al. 2007 proved the EFEMP1 mutation to be pathological by showing EFEMP1 R345W knock in mice develop deposits between Bruch's membrane and retinal pigment epithelium (RPE). They stated that the deposits may contain increased amounts of mutated EFEMP1 proteins as well as TIMP3 protein, and that this may lead to basal deposit formation. Their research also showed that R345W knock in mice demonstrated evidence of alterations in RPE cell ultrastructure, and that basal deposits and alterations in RPE cell ultrastructure were associated with increased with increased levels of C3 compliment activation[6].
Tsai et al. 2021 compared induced pluripotent stem cells (iPSC)-derived RPEs from individuals with DHRD to their controls. There was a significant down-regulation of carboxyl esterase 1 (CES1), an enzyme responsible for exporting cholesterol by turning the ester into free cholesterol. The known mutation EFEMP1 R345W in DHRD may have a hyper-inhibitory effect on EGFR signaling and suppress CES1. This decreased ability to mediate cholesterol efflux could result in the lipid accumulation found in drusen in AMD.[7]
Doyne Honeycomb dystrophy represents a unique heritable macular retinal dystrophy, in which the drusen that form and sequelae including geographic atrophy and choroidal neovascularization closely represent age-related macular degeneration, thus making EFEMP1 an important protein in the study of AMD pathogenesis, modeling, and therapeutic studies.
Diagnosis
Diagnosis of Doyne Honeycomb Retinal Dystrophy is made clinically and must be confirmed with genetic testing to prove a EFEMP1 mutation.
History and Symptoms
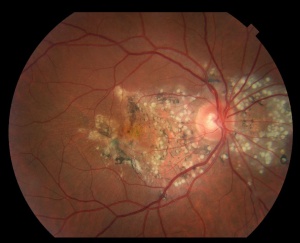
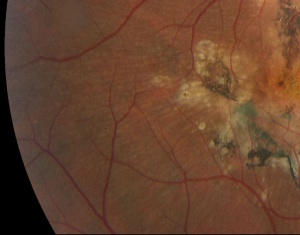
While there have been reports of disease onset in children and elderly, patients are usually asymptomatic early in the course of disease, with most not experiencing symptoms until the 4th and 5th decade of life. Patients may describe an initial insidious onset of visual disturbances such as blurry vision, photopsias, scotomas, metamorphopsias, and dyschromatopsias. In advanced stages, in the 7th and 8th decades of life, central vision is involved, and visual acuity can be worse than 20/200. Such visual changes are associated with the appearance of drusen between the Bruch membrane and RPE, that initially present as small accumulations and progress to the honeycomb pattern. Large differences in severity and progression can be seen between eyes of the same patient [8][9], as well as between patients in the same family[9]. Early onset drusen appear in a radial distribution centered on the macula, as well as the optic disk margin. More advanced and later onset disease presents with central vision loss depending on the presence or absence of choroidal neovascular membranes (CNVM) and geographic atrophy[10].
Physical Examination
The disease is typically characterized by early-onset drusenoid deposits presenting at the posterior pole, peripapillary area, center of the macula, and along vascular arcades. The deposits are characteristically described as multiple "radially elongated" or "honeycomb pattern" small drusen in early stages, but can become indistinguishable from deposits in advanced AMD in later stages as they become larger and more confluent. Involvement nasal to optic disc is a typical feature. Pigmentary changes often occur as the disease progresses . CNVM can form, with subsequent scarring, and in advanced stages geographic atrophy can occur in the areas of confluent drusen. Early stages may have bilateral symmetric basal laminar drusen.
Imaging
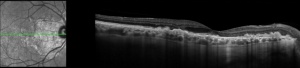
Spectral domain optical coherence tomography (SD-OCT) imaging can reveal focal dome-shaped, saw-tooth, or diffuse hyperreflective deposits with elevation between the RPE and Bruch's membrane, usually becoming more confluent over time[11]. Early in disease, the outer retina (photoreceptors) may remain intact, but later stages can show variable or diffuse ellipsoid zone loss, as well as outer and inner segment disruption. OCT can also reveal hypo-reflective fluid from a corresponding neovascular membrane. Diffuse retinal atrophy can be seen in later stages, however outer retinal changes are not often seen outside of areas with sub-RPE changes[12].
Fundus autofluorescence (FAF) can reveal hypo or hyper-autofluorescence of drusen, with one study showing larger drusen were more typically hyper-autofluorescent [11]. This increased autofluorescence associated with drusen in DHRD (and in other genetic drusen forming maculopathies) seems to be in contrast to typical drusen of AMD, where there is generally little correspondence between distribution of drusen and FAF[11][13]. Central areas may be hypo-autofluorescent due to central geographic atrophy and loss or dysfunction of RPE.
Fluoroscein angiography (FA) can reveal presence of CNVM with leakage, and large drusen are typically hyper-fluorescent in late stages[11]. Variable hypo or hyperfluorescence can be seen in areas of pigmentary changes, atrophy, and scarring. Indocyanine green angiography (ICG) can also be used to differentiate between two types of drusen. This imaging modality has shown different patterns of staining between large central drusen and small radial drusen. Large central drusen initially present as hypofluorescent, and then become hyperfluorescent spots surrounded by halos of hypofluorescence in the late phases. Small radial drusen on the other hand, present with hyperfluorescence in early phases and decreased fluorescence in late phases. [14]
OCT-A is also a useful imaging modality that can be used in visualization of DHRD. Choriocapillaris segmentation of OCT-A in DHRD patients has shown a hyperflow signal corresponding to active choroidal neovascularization. [15]
Genetic Testing
Patients with suspected DHRD or any early onset maculopathy should be considered for genetic testing, which can confirm mutation of the EFEMP1 gene. There are several commercially available genetic tests for inherited retinal diseases and dystrophies. A list of these tests can be found via the NCBI genetic testing registry below.
https://www.ncbi.nlm.nih.gov/gtr/conditions/C0854723/
Differential diagnosis
Differential diagnosis of any patient with early-onset drusen should include any of the hereditary maculopathies and pattern dystrophies:
- Stargardt Disease
- Autosomal Dominant Stargardt-Like Macular Dystrophy
- North Carolina Macular Dystrophy
- Sorsby Fundus Dystrophy: Fully penetrant autosomal dominant disease caused by variants in the tissue inhibitor gene metalloproteinases-3 (TIMP3) forming drusen like deposits at the BM and later CNV, usually sparing the peripheral retina. Patients may have difficulty with dark and color vision prior to losing central vision and usually experience severe vision loss in their 4th and 5th decades of life. Intravitreal VEGF injections are used to delay visual loss.[16]
- Best Disease
- Pattern Dystrophies
- Age-related Macular Degeneration (if presenting at more advanced age)
Management
Currently, there is no genetic or targeted therapies to correct the underlying EFEMP1 genetic mutation in DHRD.
Typically, patients with DHRD are managed conservatively with observation, unless a CNVM develops.
CNVM in DHRD is typically treated with a series of intravitreal anti-VEGF injections[17]. Anti-VEGF injections such as Bevacizumab have been shown to improve vision and resolve subretinal fluid. [18]
Another treatment option is the use of lasers to clear drusen deposits. One study showed that low-energy argon laser treatment improved visual acuity and retinal sensitivity, and decreased drusen volume. Additionally, another case report showed functional improvement using sub threshold retinal laser in a patient with DHRD [19][20].
In Cusumano, et al. 2019, sub-threshold nanolaser treatment was performed in a patient with DHRD demonstrating visual acuity and multifocal electroretinogram improvement at the 2 and 6 month mark.[21] A case series of 3 patients with DHRD by Cusumano, et al. 2023 demonstrated the potential for long-term (up to 30-months) stability and improvement to visual acuity and multifocal electroretinogram following nanosecond laser treatment[22].
Patients with a confirmed EFEMP1 mutation should have their children screened for involvement.
Follow up
We recommend annual follow up with OCT imaging in patients without CNVM.
Prognosis
Prognosis is variable, not only between patients but also between the eyes of the same patient. Central visual acuity can be excellent, but generally worsens with age, with vision less than 20/200 in the 7th and 8th decades of life not being uncommon in some cases.
Additional Resources
Online Mendelian Inheritence in Man https://www.omim.org/entry/126600
References
- ↑ Doyne, R.W. Peculiar condition of choroiditis occurring in several members of the same family. Trans. Ophthalmol. Soc. UK 19, 71 –71 (1899).
- ↑ Vogt, A. in Handbuch der gesammten Augenheilkunde. Untersuchungsmethoden (eds Graefe, A. & Saemisch, T.) 1–118 (Verlag von Wilhelm Engelman, Berlin, 1925).
- ↑ Héon, Elise, et al. "Linkage of autosomal dominant radial drusen (malattia leventinese) to chromosome 2p16-21." Archives of ophthalmology 114.2 (1996): 193-198.
- ↑ Gregory, Cheryl Y., et al. "The gene responsible for autosomal dominant Doyne's honeycomb retinal dystrophy (DHRD) maps to chromosome 2p16." Human molecular genetics 5.7 (1996): 1055-1059.
- ↑ Stone, Edwin M., et al. "A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy." Nature genetics 22.2 (1999): 199-202.
- ↑ Fu, Li, et al. "The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice." Human molecular genetics 16.20 (2007): 2411-2422.
- ↑ Tsai, et al. (2021).“Impaired cholesterol efflux in retinal pigment epithelium of individuals with juvenile macular degeneration.” The American Journal of Human Genetics. 108(3), 903-918.
- ↑ ZECH, JEAN-CHRISTOPHE, et al. "Macular dystrophy of malattia leventinese. A 25 year follow up." British journal of ophthalmology 83.10 (1999): 1194-1194.
- ↑ Jump up to: 9.0 9.1 Michaelides, Michel, et al. "Maculopathy due to the R345W substitution in fibulin-3: distinct clinical features, disease variability, and extent of retinal dysfunction." Investigative ophthalmology & visual science 47.7 (2006): 3085-3097.
- ↑ Zhang, T., Xie, X., Cao, G., Jiang, H., Wu, S., Su, Z., ... & Lu, F. (2014). Malattia leventinese/Doyne honeycomb retinal dystrophy in a Chinese family with mutation of the EFEMP1 gene. Retina, 34(12), 2462-2471. (13)
- ↑ Jump up to: 11.0 11.1 11.2 11.3 Querques, Giuseppe, et al. "Multimodal morphological and functional characterization of Malattia Leventinese." Graefe's Archive for Clinical and Experimental Ophthalmology 251.3 (2013): 705-714.
- ↑ Gerth, C., Zawadzki, R. J., Werner, J. S., & Heon, E. (2009). Retinal microstructure in patients with EFEMP1 retinal dystrophy evaluated by Fourier domain OCT. Eye, 23(2), 480-483.
- ↑ Michaelides, Michel, et al. "Maculopathy due to the R345W substitution in fibulin-3: distinct clinical features, disease variability, and extent of retinal dysfunction." Investigative ophthalmology & visual science 47.7 (2006): 3085-3097.
- ↑ Souied, E. H., Leveziel, N., Querques, G., Darmon, J., Coscas, G., & Soubrane, G. (2006). Indocyanine green angiography features of Malattia leventinese. British Journal of Ophthalmology, 90(3), 296-300.
- ↑ Serra R, Coscas F, Messaoudi N, Srour M, Souied E. Choroidal Neovascularization in Malattia Leventinese Diagnosed Using Optical Coherence Tomography Angiography. Am J Ophthalmol. 2017 Apr;176:108-117. doi: 10.1016/j.ajo.2016.12.027. Epub 2017 Jan 11. PMID: 28088509.
- ↑ Cho, I.H. (2022). Other Macular Dystrophies 2. In: Yu, HG. (eds) Inherited Retinal Disease. Springer, Singapore.
- ↑ Sohn, Elliott H., et al. "Responsiveness of Choroidal Neovascular Membranes in Patients With R345W Mutation in Fibulin 3 (Doyne Honeycomb Retinal Dystrophy) to Anti–Vascular Endothelial Growth Factor Therapy." Archives of ophthalmology 129.12 (2011): 1626-1628.
- ↑ Parameswarappa, D. C., & Rani, P. K. (2021). Utility of pattern recognition and multimodal imaging in the diagnosis and management of doyne honeycomb retinal dystrophy complicated with type one choroidal neovascular membrane. BMJ Case Reports CP, 14(2), e237635.
- ↑ https://link.springer.com/article/10.1186/s13256-018-1935-1
- ↑ Lenassi, E., Troeger, E., Wilke, R., Tufail, A., Hawlina, M., Jeffery, G., & Webster, A. R. (2013). Laser clearance of drusen deposit in patients with autosomal dominant drusen (p. Arg345Trp in EFEMP1). American Journal of Ophthalmology, 155(1), 190-198.
- ↑ Cusumano A, Falsini B, Giardina E, Cascella R, Sebastiani J, Marshall J. Doyne honeycomb retinal dystrophy - functional improvement following subthreshold nanopulse laser treatment: a case report. J Med Case Rep. 2019 Jan 10;13(1):5.
- ↑ Cusumano A, Falsini B, D'Ambrosio M, D'Apolito F, Sebastiani J, Levialdi Ghiron JH, Giardina E, Cascella R. Long-Term Structural and Functional Assessment of Doyne Honeycomb Retinal Dystrophy following Nanosecond 2RT Laser Treatment: A Case Series. Case Rep Ophthalmol. 2023 Nov 23;14(1):626-639. doi: 10.1159/000534579. PMID: 38023612; PMCID: PMC10666958.