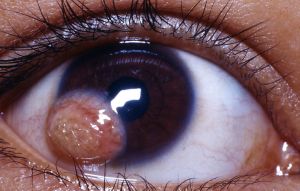
Corneal Dermoid
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Corneal dermoid
ICD-11: 2F36.Y
Disease
A corneal dermoid is a benign congenital choristoma (growth of microscopically normal tissue in an abnormal location) on the anterior surface of the eye. It is also called limbal dermoid and epibulbar demoid. It frequently presents as a whitish or yellowish lesion at the corneal limbus, though limbus-sparing dermoids and dermoids involving the entire cornea have been reported.[1][2] Importantly, corneal dermoids and orbital dermoid cysts are two separate disease entities.
Risk Factors & Associated Conditions
Several systemic conditions and other syndromes have been associated with corneal dermoids.
Goldenhar syndrome
Also called oculo-auriculo-vertebral spectrum and facio-auriculo-vertebral dysplasia, Goldenhar syndrome is characterized by the classic triad of mandibular hypoplasia resulting in facial asymmetry, ocular and auricular malformations, and vertebral anomalies. Ocular malformations may include corneal dermoids, microphthalmia, and coloboma. Most cases of Goldenhar syndrome are sporadic, but familial cases have been reported.[3]
Ring dermoid syndrome
Ring dermoid syndrome is a rare syndrome with autosomal dominant inheritance characterized by bilateral annular limbal dermoids.[4][5] Additional clinical findings include conjunctival keratinization, hairs, and corneal lipid deposition. No extraocular anomalies have been reported. Mutations in PITX2 have been associated with ring dermoid syndrome.[5]
Epidermal nevus syndromes
Epidermal nevus syndromes are a group of rare disorders characterized by the presence of skin lesions known as epidermal nevi and extra-cutaneous abnormalities, most often affecting the brain, eye, and skeletal systems. Ocular abnormalities may include corneal dermoids, cataracts, and colobomas. Of note, sebaceous nevus syndrome, a type of epidermal nevus syndrome, is known to have malignant potential.[6]
Other associations
Corneal dermoids may present with a variety of ocular abnormalities and extraocular abnormalities. Examples include scleral/corneal staphyloma, aniridia, aphakia, cataract, microphthalmia, coloboma of the upper eyelid, lacrimal stenosis, nevus flammeus, neurofibromatosis, Treacher Collins syndrome, and Duane syndrome.
General Pathology
Corneal dermoids are solid (not cystic) choristomas with surface epithelium resembling epidermis and dermis, often containing collagenous connective tissue, hair, skin, fat, and sebaceous glands on histology. Grossly, they are elevated, opaque, yellow-white masses often localized to the corneal limbus.
Pathophysiology
Several theories about the development of corneal dermoids exist. One theory proposes that corneal dermoids arise from metaplastic transformation of the mesoblast between the rim of the optic nerve and the surface ectoderm in early development. Another theory suggests sequestration of the pluripotential cells during embryonic development of the surrounding ocular structures. The exact pathogenesis likely varies case by case.
Most cases of corneal dermoids are sporadic, though familial presentations of corneal dermoids have been reported especially in the context of Goldenhar syndrome and ring dermoid syndrome.[3][4]
Diagnosis
The diagnosis of a corneal dermoid is largely clinical. Biopsy is generally not necessary. However, if surgical removal of the lesion is pursued, histopathology can confirm the diagnosis.
History
Patients with corneal dermoids usually present early in life, as the condition is congenital. Personal or family history of facial syndromes or ocular conditions may aid in the diagnosis. Common presenting complaints include decreased vision, foreign body sensation, cosmetic disfigurement, and enlarging ocular mass.
Physical Examination
In addition to a comprehensive eye examination, a physical examination for non-ocular malformations is helpful due to the association of corneal dermoids with systemic conditions and other syndromes. In the case of Goldenhar syndrome, head and neck examination may reveal periauricular tragi, microtia or anotia of external ear, hearing loss, low implantation of the auricular pavilion, micrognathia, or mandibular hypoplasia. Vertebral anomalies are also common, such as scoliosis and hemivertebrae. Congenital heart disease and central nervous system abnormalities have also been reported.
Ocular examination
Visual acuity measurement is important to help diagnose and manage possible amblyopia ipsilateral to the dermoid. Additionally, the size of the lesion, ideally captured and measured by digital photography, sensorimotor examination, and cycloplegic refraction are important to assess. If an in-office examination is not feasible, examination under anesthesia should be done for surgical planning.
Most corneal dermoids are located at the inferior temporal limbus, though they could affect only the cornea. The dermoid usually has a dome shape, visible hair follicles, and cilia. The dermoid is often vascularized.
Other ocular abnormalities that may be noted on examination include coloboma of the eyelids, Duane retraction syndrome, other ocular motility disorders, lacrimal anomalies, scleral and corneal staphylomata, aniridia, and microphthalmia.
Diagnostic procedures
Anterior segment ultrasound biomicroscopy could be used to assess the depth of the dermoid, particularly the involvement of Descemet membrane. However, Hoops et al have shown that dermoids produce strong sound attenuation, making it challenging to visualize Descemet membrane.[7] Anterior segment optical coherence tomography serves as an alternative imaging technique. Its clinical utility in comparison with ultrasound biomicroscopy remains unclear.[8]
Differential diagnosis
- Peters anomaly
- Juvenile xanthogranuloma
- Sclerocornea
- Corneal scar
- Pterygium
- Foreign body granuloma
Management
Corneal dermoids have been traditionally classified into three grades to help guide clinical and surgical management. Grade I corneal dermoids are superficial lesions measuring less than 5mm and localized to the limbus. Grade II corneal dermoids are larger lesions covering most of the cornea and extending deep into the stroma down to Descemet membrane without involving it. Grade III corneal dermoids are large lesions covering the whole cornea and extending through the structures between the anterior surface of the eye and the pigmented epithelium of the iris. In general, only small asymptomatic Grade I dermoids may be medically managed.
Medical management
Medical management is appropriate for Grade I dermoids that induce only mild astigmatism of < 1D with minimal surface irregularity and good patient adherence to spectacle correction. In these cases, close clinical observation with serial examinations in clinic is recommended. The serial examinations should be performed once every 6-12 months and should include visual acuity, measurement of the lesion, stereopsis, and cycloplegic refraction. In the presence of suspected or proven amblyopia, vision should be monitored every 4 months, and treatment with spectacles and occlusion therapy should be attempted. Surgery carries the risk of postoperative scarring and development of pseudopterygium.
Surgical management
Surgery is indicated in patients with grade I limbal dermoids if they have the following conditions.
- Chronic eye rubbing due to irritation and recurrent conjunctivitis
- Amblyopia unresponsive to medical management
- Progressive dellen with corneal surface decompensation
- Growth and encroachment into pupillary area or optical zone
- Aesthetic considerations
- Inadequate lid closure
Surgery is universally indicated for grade II and III limbal dermoids.[9]
A variety of surgical approaches to corneal dermoids have been described in the literature. A comprehensive review of the literature by Pirouzian generated the following recommendations[9]:
- Grade I, <50 μm thickness and <1 mm diameter*: simple excision
- Grade I, <100 μm thickness and <1 mm diameter*: keratectomy + amniotic membrane transplantation + autologous limbal stem cell allograft
- Grade II and deeper Grade I: keratectomy + amniotic membrane transplantation + limbal stem cell allograft + pericardial patch graft versus anterior or deep anterior lamellar keratoplasty ± amniotic membrane transplantation
- Grade III: total anterior segment reconstruction
*Corneal dermoids of such small size were rarely observed in our clinical practice.
Additionally, optical iridectomy is frequently needed in staged surgical management to permit visual development in the absence of clear cornea.
Complications
- Persistent epithelial defect
- Corneal vascularization
- Corneal scarring
- Pseudopterygium
- Incomplete excision
- Globe perforation
- Postoperative astigmatism
- Graft failure
- Graft rejection
- Steroid-induced glaucoma
- Infection
Prognosis
A validated grading system has been developed to prognosticate visual outcomes in patients with corneal dermoids. The system classifies dermoids based on area of corneal involvement, surface shape, and area of conjunctival involvement.[10] In general, lower-grade dermoids carry better visual prognosis. Surgical intervention of corneal dermoids may allow for good functional, refractive, and cosmetic outcomes. However, a 10-year case series on limbal dermoids reports that amblyopia not responsive to medical management also did not respond to surgical management.[11]
References
- ↑ Hameed S, Kaur I, Singh V, Mishra DC, Reddy JC. Congenital central corneal dermoid: A rare entity. European Journal of Ophthalmology. 2022;32(3):NP5-NP9. doi:10.1177/1120672120986365
- ↑ Liu J, Liang L. A giant corneal dermoid. BMJ. 2018;362:k3200. doi:10.1136/bmj.k3200
- ↑ Jump up to: 3.0 3.1 Baum JL, Feingold M. Ocular Aspects of Goldenhar’s Syndrome. American Journal of Ophthalmology. 1973;75(2):250-257. doi:10.1016/0002-9394(73)91020-9
- ↑ Jump up to: 4.0 4.1 Mattos J, Contreras F, O’Donnell FE. Ring dermoid syndrome. A new syndrome of autosomal dominantly inherited, bilateral, annular limbal dermoids with corneal and conjunctival extension. Arch Ophthalmol. 1980;98(6):1059-1061. doi:10.1001/archopht.1980.01020031049007
- ↑ Jump up to: 5.0 5.1 Xia K, Wu L, Liu X, et al. Mutation in PITX2 is associated with ring dermoid of the cornea. Journal of Medical Genetics. 2004;41(12):e129-e129. doi:10.1136/jmg.2004.022434
- ↑ Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1(6):545-556. doi:10.1016/s0190-9622(79)80100-0
- ↑ Hoops JP, Ludwig K, Boergen KP, Kampik A. Preoperative evaluation of limbal dermoids using high-resolution biomicroscopy. Graefes Arch Clin Exp Ophthalmol. 2001;239(6):459-461. doi:10.1007/s004170100300
- ↑ Janssens K, Mertens M, Lauwers N, de Keizer RJW, Mathysen DGP, De Groot V. To Study and Determine the Role of Anterior Segment Optical Coherence Tomography and Ultrasound Biomicroscopy in Corneal and Conjunctival Tumors. J Ophthalmol. 2016;2016:1048760. doi:10.1155/2016/1048760
- ↑ Jump up to: 9.0 9.1 Pirouzian A. Management of pediatric corneal limbal dermoids. Clin Ophthalmol. 2013;7:607-614. doi:10.2147/OPTH.S38663
- ↑ Zhong J, Deng Y, Zhang P, et al. New Grading System for Limbal Dermoid: A Retrospective Analysis of 261 Cases Over a 10-Year Period. Cornea. 2018;37(1):66-71. doi:10.1097/ICO.0000000000001429
- ↑ Yao Y, Zhang MZ, Jhanji V. Surgical management of limbal dermoids: 10-year review. Acta Ophthalmol. 2017;95(6):e517-e518. doi:10.1111/aos.13423