Biologics for Thyroid Eye Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Thyroid Eye Disease Overview
Graves’ Disease (GD) or thyroid endocrinopathy is characterized by an autoimmune activation of the thyrotropin receptor (TSHR). Thyroid receptor antibodies (TRAbs) stimulate the THSR in thyrocytes, mimicking TSH causing hyperthyroidism. Thyroid eye disease (TED) or Graves’ orbitopathy is an autoimmune-driven ophthalmic presentation affecting about 25% of patients with GD. However, thyroid eye disease may present independently of GD or autoantibodies and is thought to be a more complex disease process involving genetic and environmental factors (Lane, Cheetham, Perros, & Pearce, 2020).[1]
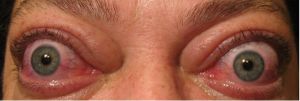
The ophthalmic manifestations of TED include periorbital edema, erythema, proptosis, eyelid retraction, restrictive strabismus leading to diplopia, chemosis, and in more severe cases, increased intraocular pressure, exposure keratopathy and even optic nerve compression (Figure 1A, 1B). These symptoms can have a profound physical and psychological effect. Additionally, TED may affect CNS function and cerebral cortical thickness (Silkiss & Wade, 2016).[2]
Both antigen-specific (autoimmune) and antigen-independent (autoinflammatory) pathways play a role in TED, though the presence of autoantibodies implicates both cell-mediated and humoral immunity. TED most often manifests when patients are hyperthyroid, though it may present when patients are hypothyroid or even euthyroid and can occur concurrently with endocrine abnormalities or may precede or follow them (Lane et al., 2020).[1]
Until 2010 with interest in and trials of Rituximab for TED (Silkiss, Reier, Coleman, & Lauer, 2010), (Hegedüs, Smith, Douglas, & Nielsen, 2011)[3][4] management involved supportive treatment with or without immunosuppression (steroids), as well as radiation therapy, eyelid malposition surgery, and surgical decompression. Non-pharmacologic therapies including eye lubrication, prisms for diplopia, smoking cessation, and oral selenium were recommended. The advent of immunotherapies for TED has altered the medical treatment of this disease.
Rituximab
Despite TED being commonly associated with T-cell immunity, B cells provide a potential immunologic treatment target. B cells are implicated in both production of TRAbs and antigen presentation, specifically presentation of TSHR to T-cells which further produce pro-inflammatory cytokines that stimulate muscle and adipose proliferation (Hegedüs et al., 2011) (Lane et al., 2020).[4][1] B cells also play a role in the persistence of TRAbs after thyroidectomy. In the thyroid, thyrocytes develop lymphocytic infiltrates consisting of T and B cells, which may form intrathyroidal germinal centers that contribute to TRAbs-producing plasma cells (El Fassi, Clemmensen, Nielsen, Silkiss, & Hegedüs, 2007).[5]
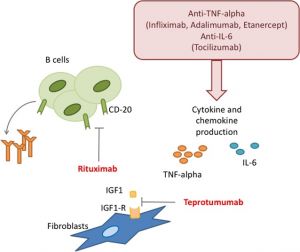
Rituximab is an anti-CD20 monoclonal antibody originally used to treat lymphoproliferative malignancies and increasingly used to treat autoimmune conditions. It acts on B cells to prevent activation and differentiation through a variety of mechanisms not entirely understood (Lane et al., 2020).[1]
It works independently of TSHR and IGFR1, suggesting an antigen-presenting mechanism (Douglas & Gupta, 2011).[6] CD20 is not expressed on plasma cells. This explains why patients can still mount an immune response after rituximab therapy (Lane et al., 2020).[1] Several studies have shown promising results from rituximab in TED. In a 2007 case report of a patient with GD, rituximab completely depleted non-circulating intrathyroidal B cells (El Fassi, Clemmensen, Nielsen, Silkiss, Hegedüs 2007).[5] A 2015 randomized control trial of 32 patients found that rituximab was effective in lowering clinical activity scores (CAS) by at least 2 points compared to IV methylprednisolone, without evidence of disease recurrence at 24 weeks (Salvi et al., 2015).[7] Another interventional trial found that CAS scores were reduced at 1 year with rituximab, though it was unclear whether this followed the natural disease course. However, in two separate studies there was no recurrence of active disease despite elevated TSI and a return to normal B-cell levels, suggesting rituximab may modulate disease progression independent of humoral-mediated response. This data corroborated an antigen-presenting feature of B cells (Salvi, Vannucchi, Campi, & Beck-Peccoz, 2009), (Silkiss et al., 2010).[8][3] Even in corticosteroid-resistant patients, rituximab has been shown to be effective in reducing CAS and preventing disease recurrence (Khanna et al., 2010).[9] Low-dose rituximab was incidentally shown to be beneficial in preventing disease recurrence in three patients, discovered after stopping the infusion after 100 mg due to adverse reactions in two of them. Regardless, there is more research to be done to study altered dosing of rituximab to reduce side effects and cost by administering lower doses with similar efficacy (Salvi et al., 2012).[10]
Not all studies have found rituximab to be effective, as evidenced by a 2015 study of 25 patients, in which eight patients on rituximab developed adverse events, compared to three in the placebo group (Stan et al., 2015).[11] As such, further clinical research is necessary to investigate the safety and efficacy of rituximab in TED. Specifically, larger randomized, controlled trials are needed, as most studies of rituximab are comprised of small, heterogeneous cohorts (Hegedüs et al., 2011).[4] Additionally, rituximab should be studied for efficacy in patients in the active phase of their disease, similar to the teprotumumab study. In several prior rituximab studies, specifically Stan et al, the disease was well past its active phase when given to patients. A 2021 meta-analysis of fifteen RCTs comparing rituximab and teprotumumab, among other treatment modalities, against placebo, found that rituximab was not found to be significantly more effective than IVGCs in reducing proptosis or diplopia, though it remains a second-line treatment for active and moderate-to-severe refractory thyroid eye disease (Li et. al, 2021).[12]
Less studied is Novartis’ iscalimab, an anti-CD40 monoclonal antibody which blocks CD40-CD154 binding without depleting B cells. It has been shown to reduce TRAbs in 15 patients with GD, two of whom also had improvement in eye symptoms (Kahaly et al., 2020).[14]
Tocilizumab
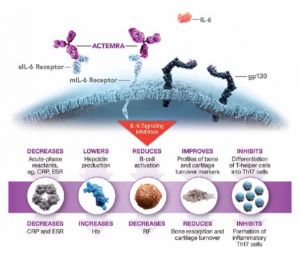
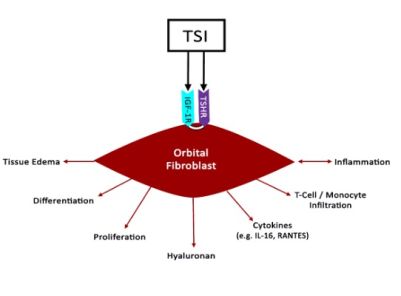
Tocilizumab (Actemra, Genentech, South San Francisco) is a monoclonal antibody to IL-6. IL-6 stimulates expression of TSHR in orbital fibroblasts (Figure 4). Tocilizumab is FDA-approved to treat rheumatoid arthritis, juvenile idiopathic arthritis, cytokine release syndrome, giant cell arteritis. In May 2021, tocilizumab was approved to treat systemic sclerosis-associated interstitial lung disease stabilizing pulmonary function tests (Roofeh et al., 2021).[15] A 2021 systematic review of two randomized controlled trials suggests tocilizumab may be beneficial in promoting sustained remission, relapse-free survival, and decreasing the need for escape therapy (Antonio, Santos, & Abariga, 2021).[16] A 2021 meta-analysis found that tocilizumab may also be beneficial in reducing mortality in severe COVID-19 infections, dampening the inflammatory cytokine storm, though robust evidence from randomized controlled trials is lacking (Sarfraz, et al, 2021).[17]
In patients with moderate-severe thyroid eye disease, resistant to corticosteroids, tocilizumab decreases CAS compared to placebo at 16 weeks (93% versus 59%), without significant difference at 40 weeks. Proptosis was reduced by at least two millimeters in 78% of patients in this study. Proptosis reduction was stratified by baseline measurements, with mean reduction of 1.2mm for exophthalmos less than or equal to 20mm, 2.4mm for 21-22mm, 2.7mm for 23-24, and 3.8mm reduction for eyes with baseline measurement greater than or equal to 25mm (Perez-Moreiras et al., 2018).[18] While it has been associated with a higher recurrence rates and adverse effects such as hypercholesterolemia, neutropenia, and transaminitis (Sears CM, 2020)[19], a 2020 multicenter observational study of 48 patients found that tocilizumab significantly improved CAS, visual acuity, and intraocular pressure in patients without any serious adverse effects (Sánchez-Bilbao et al., 2020).[20] Tocilizumab has also been shown to be effective when administered subcutaneously during which it effectively lowers TSI levels (Silkiss, Paap, Roelofs, Agi, & Weis, 2021).[21]
Teprotumumab
Stimulation of TSHR by thyroid stimulating immunoglobulins (TSI) and autoantibodies to the insulin-like growth factor 1 receptor (IGF-1R) are thought to work synergistically to activate orbital fibroblasts and excessive hyaluronan production in TED (Figure 2). Both TSI and IGFR1 have shared epitopes (Douglas & Gupta, 2011).[6] Compared to healthy control orbits, TED patients have increased expression of TSHR and IGF-1R. Furthermore, GD-associated antibodies were found to stimulate orbital fibroblasts derived from GD donor tissue to synthesize inflammatory cytokines mimicking IGF-1R stimulation (Tsui et al., 2008), (Pritchard, Han, Horst, Cruikshank, & Smith, 2003).[22][23] When either TSHR or IGF-1R are stimulated on fibroblasts, both receptors co-localize to cell membranes, providing further evidence that IGF-1R mediates TSH signaling (Tsui et al., 2008).[22] Together, these findings implicate TSHR and IGF-1R as co-conspirators in TED progression.
Teprotumumab (Tepezza, Horizon Therapeutics, Deerfield, Illinois) is currently the only FDA-approved immunotherapy for thyroid eye disease. Teprotumumab is a monoclonal antibody against IGF-1R which was initially shown in-vitro to reduce TSH signaling in fibrocytes (Chen et al., 2014).[24] Teprotumumab demonstrated, in two small multicenter, randomized, double-blinded trials significant reduction of proptosis (mean reduction of 2.82 millimeters, with 83% of patients having a proptosis reduction of at least two millimeters) and CAS (Smith et al., 2017), (Douglas et al., 2020).[25][26] A 2021 meta-analysis reviewed fifteen RCTs comparing teprotumumab and rituximab, among other treatment modalities, against placebo, and found that teprotumumab was more effective in reducing proptosis than intravenous glucocorticoids (IVGCs), though there was no significant difference in diplopia. Overall, Teprotumumab was found to be the most efficacious treatment in terms of overall response rate. (Li et al., 2021).[12]
Because IGF-1 is expressed systemically, teprotumumab can have a wide variety of adverse effects. These include muscle spasms, alopecia, gastrointestinal symptoms, hearing impairment, hyperglycemia (Douglas et al., 2020).[26] Even encephalopathy has been reported (Hoang, Nguyen, Chou, & Shakir, 2021).[27] The incidence of side effects is significant, and in some cases irreversible. In a multicenter observational study, 78% of the 51 patients developed adverse effects, 30% of whom had persistent symptoms at last follow up (Sears et al., 2021).[28] Despite this, treatment to completion rates remain high; a recent observational study found 90% adherence. Of the 10% who were unable to finish their prescribed regimen, the majority stopped due to adverse effects (Douglas et al., 2021).[29]
Research IGF-1R Inhibitors
Viridian Therapeutics’ VRDN-001, is an anti-IGF-1R monoclonal antibody in development for TED. This antibody was previously studied in over 100 oncology patients; the pharmacokinetic, pharmacodynamic, safety, and tolerability data from that program informed the development plans for VRDN-001.
Viridian began developing VRDN-001 for all non-oncology indications that do not use radiopharmaceuticals, including treatment of TED. In October 2020, the company submitted an investigational new drug application to the FDA to begin phase I/II clinical trials in patients with TED. In-vitro studies have demonstrated high potency IGF-1R inhibition and suggest favorable clinical efficacy; it is administered intravenously. Clinical data announcement is expected in the second quarter of 2022.
VRDN-002, a second-generation version, features an extended half-life, allowing for more stable steady-state concentrations as well as subcutaneous administration. In primates, this drug demonstrated favorable pharmacokinetics, suggesting potential use for a more convenient treatment of TED. The pharmacodynamics of this drug may lead to reduced side effects as a blunted Cmax may have less impact on the IGF-1R sensitive auditory and other systems.
FcRn Inhibitors
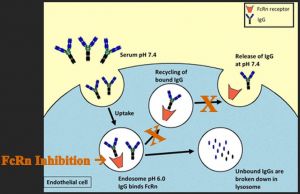
Another immunologic target for the treatment of TED is the neonatal Fc receptor (FcRn), which is responsible for the passive transfer of humoral immunity from mother to neonate and engages in immunoglobulin recycling by transportation of immunoglobulins back to the cell membrane for release after endocytosis (Roopenian & Akilesh, 2007).[30]
Inhibition of FcRn can reduce immunoglobulin recycling and decrease circulating levels of antibodies (Figure 3). An experimental drug in development by Immunovant, Inc, New York, NY, batoclimab (IMVT-1401), limits the FcRn and demonstrates promising results in phase II clinical trials for TED. An advantageous element of this drug is that is administered subcutaneously and thus could be given at home.
However, Immunovant recently paused all phase II trials for batoclimab in various autoimmune conditions due to dose-dependent hypercholesterolemia in study participants, which resolved at 20 weeks. No adverse cardiovascular events were reported. Interestingly, ongoing studies of IMVT-1401 in China in patients with myasthenia gravis (MG) and idiopathic thrombocytopenic purrpura (ITP) did not have similar elevated cholesterol levels. Recently, a phase III trial has begun in China for patients with MG. Immunovant reports restarting its US clinical trials of batoclimab in various diseases at the end of 2021 or early 2022.
Four other similar anti-FcRn drugs include Argenx US (Boston, Massachusetts) efgartigimod, UCB Pharma (Brussels, Belgium), rozanolixizumab, Momenta Pharmaceuticals (recently acquired by Janssen, Titusville, New Jersey), nipocalimab, and Alexion (Boston, Massachusetts) ALXN-1830, However, these have currently not been evaluated in GD or TED.
Efgartigimod is an antibody fragment that blocks FcRn and was found to significantly improve functional scores on the MG-ADL scale in a phase III clinical trial of patients with myasthenia gravis (MG). Efgartigimod has a strong safety profile, with rates of adverse effects similar between treatment and placebo arms. Efgartigimod is currently available in intravenous form, though Argenx is evaluating subcutaneous administration. While this drug has been studied only in MG, the company is exploring its efficacy across a wide range of IgG-mediated autoimmune conditions, including thyroid eye disease.
Rozanolixizumab is a monoclonal antibody against FcRn that has been studied in phase II trials for MG and ITP with promising results. In ITP studies, rozanolixizumab demonstrated improved platelet counts and decreased serum IgG levels, with mild-moderate headaches as the primary adverse effect and no patients dropping out of the study (Robak et al., 2019).[31] In MG, rozanolixizumab was not shown to significantly improve patients’ quantitative myasthenia gravis (QMG). Rozanolixizumab demonstrated higher rates of headaches in MG patients than efgartigimod. However, the data overall suggest there may be clinical benefit in MG of this class of antibody and phase III trials are ongoing (Bril et al., 2021).[32] This drug is also administered subcutaneously.
Momenta Pharmaceuticals’ nipocalimab is an anti-FcRn monoclonal antibody in phase III trials for patients with MG. Nipocalimab, an intravenously administered monoclonal antibody against FcRn, demonstrated in phase II trials significant improvement MG-ADL scores with no severe adverse events. Nipocalimab is also being studied in warm autoimmune hemolytic anemia and hemolytic anemia of the newborn and fetus, as it was noted to cross the placenta. Its use may be applied to TED in the future.
Alexion AstraZeneca Rare Disease has developed a similar drug, orlanolimab (ALXN-1830), development of which was initially halted after unfavorable early data amidst the start of the COVID-19 pandemic. Its phase II studies in patients with MG and warm autoimmune hemolytic anemia were restarted earlier this year. This drug is administered subcutaneously.
Others
Other molecular targets for the management of TED include tumor necrosis factor-alpha (TNF-alpha), and TSHR. Adalimumab, etanercept, and infliximab are monoclonal antibodies to TNF-alpha, which may also decrease the inflammatory response in TED. Adalimumab was found have a significant effect on patients with high baseline inflammatory composite score, but not in patients with less severe inflammatory signs (Ayabe, Rootman, Hwang, Ben-Artzi, & Goldberg, 2014).[33] Etanercept has also been shown to improve CAS in a patient with rheumatoid arthritis (RA) and GD treated with etanercept for RA (Boskovic, Medenica, Radojevic, & Zarkovic, 2019).[34] Infliximab has been reported to improve visual acuity and CAS score in sight-threatening TED (Durrani, Reuser, & Murray, 2005).[35]
TSHR apitopes, synthetic peptides that mimic CD4+ T-cell epitopes, can be used to modulate the immune response to induce self-tolerance, similar to allergy immune tolerance induction. ATX-GD-59 by Apitope Technology (University of Bristol, United Kingdom) is a mixture of two synthetic peptides of human TSHR which bind to HLA-DR molecules on dendritic cells in lymph nodes, reducing the risk of activation of antigen-presenting cells. While this apitope has been only studied in mice, successful tolerance induction was achieved and holds promise for future studies in humans with TED (Jansson, Vrolix, Jahraus, Martin, & Wraith, 2018).[36]
Conclusion
The treatment of thyroid eye disease continues to evolve with the development of new immunologic therapies. Specifically, inhibition of IGF-1R and FcRn are among the newest biologic targets with ongoing clinical trials in thyroid eye disease. CD20, TNF-alpha, and IL6 are also potential targets (Figure 5). Ongoing studies and further research are needed to optimize our understanding and treatment of thyroid eye disease with the goal of disease prevention, sign and symptom mitigation and reversal without significant side effects. No matter which biologic is used to treat the ophthalmic manifestations of thyroid disease, the systemic effects of these drugs needs to be monitored and managed proactively.
Table 1
| Drug | Mechanism of Action | Administration | Target Disease | Development Stage | Manufacturer |
|---|---|---|---|---|---|
| Tepezza
(Teprotumumab) |
Anti-IGF1R monoclonal antibody | Intravenous | Thyroid eye disease (TED) | FDA-approved | Horizon Therapeutics |
| VRDN-001 | Anti-IGF1R monoclonal antibody | Intravenous | TED | Phase I/II clinical trials | Viridian Therapeutics |
| VTERDN-002 | Anti-IGF1R monoclonal antibody (extended half-life) | Subcutaneous | TED | Phase I/II clinical trials | Viridian Therapeutics |
| IMVT-1401 (batoclimab) | Anti-FcRn monoclonal antibody | Subcutaneous | TED, Myasthenia Gravis (MG), idiopathic thrombocytopenia purpura (ITP) | Phase II clinical trials (paused in US), phase III (for MG, in China) | Immunovant |
| Vyvgart (Efgartigimod) | FcRn-blocking antibody fragment | Intravenous | MG | Phase III clinical trials | Argenx |
| Rozanolixizumab | Anti-FcRn monoclonal antibody | Subcutaneous | MG, ITP | Phase III clinical trials | UCB Pharma |
| Vivacity-MG (Nipocalimab) | Anti-FcRn monoclonal antibody | Intravenous | MG, warm autoimmune hemolytic anemia, hemolytic anemia of newborn | Phase III clinical trials | Momenta |
| ALXN-1830 (orlanolimab) | Anti-FcRn monoclonal antibody | Subcutaneous | MG, warm autoimmune hemolytic anemia | Phase II clinical trials | Alexion AstraZeneca Rare |
| Linsitnib | IGF-1R inhibitor | Oral | TED; cancer trials halted | Phase II clinical trials | Astellas Pharma |
| Lonigutamab | IGF-1R inhibitor (sub-50 pM potency and no measurable effector function) | Subcutaneous | TED | Phase I clinical trials | ValenzaBio |
| Benlysta (Belimumab) | BAFF (B cell activating factor), BLyS inhibitor | Intravenous | TED, systemic lupus erythematosus, lupus nephritis | Phase II | GlaxoSmithKline |
| Cosentyx (Secukinumab) Study terminated. Failure to meet goals. | IL-17A (cytokine, interleukin) | Subcutaneous | TED, psoriasis, psoriatic arthritis, ankylosing spondylitis | Phase II/III | Novartis |
| Eylea (Aflibercept) | VEGF inhibitor | Sub-tenon's | TED, age-related macular degeneration | Phase II | Regeneron |
Table 2
| Drug | Adverse Effects | Dosing | Cost per Dose (68 kg) | Cost per Treatment (68 kg) |
|---|---|---|---|---|
| Teprotumumab | Muscle spasms (25%)
Alopecia (13%) Hearing loss (10%) Hyperglycemia (10%) |
Initial Dose = 10mg/kg (IV)
Subsequent: 20mg/kg (IV) Total 8 doses |
$40,636 | $304,770 |
| Tocilizumab | Hypercholesterolemia (10%)
Neutropenia (10%) |
8mg / kg (IV) or 162mg (subcutaneous)
4 monthly IV doses or 8 weekly subcutaneous doses |
$3,339 (IV) or $1100 (subcutaneous) | $13,356 (IV) or $8,800(subcutaneous) |
| Rituximab | Cardiac disease
Rash Hepatitis B reactivation PML (rare) |
1000mg (IV) | $9818 | $19,636 |
Additional Resources
- https://www.evaluate.com/vantage/articles/news/trial-results/argenx-sets-high-bar-novel-drug-class
- https://www.fiercepharma.com/special-report/efgartigimod-10-most-anticipated-drug-launches-2021
- https://myastheniagravisnews.com/news-posts/2021/06/03/immunovant-planning-pivotal-trial-of-imvt-1401-in-myasthenia-gravis/
- https://www.prnewswire.com/news-releases/janssen-showcases-phase-2-nipocalimab-m281-data-in-adults-with-generalized-myasthenia-gravis-gmg-at-the-2021-american-academy-of-neurology-virtual-meeting-301270233.html
- https://www.manufacturingchemist.com/news/article_page/Therapeutic_nipocalimab_for_myasthenia_gravis/176127
- https://www.biospace.com/article/alexion-discretely-discontinues-development-of-an-anti-fcrn-drug/
- https://adisinsight.springer.com/drugs/800045005
- https://seekingalpha.com/pr/18512527-viridian-therapeutics-submits-investigational-new-drug-application-for-vrdnminus-001-igfminus
- https://drug-dev.com/viridian-therapeutics-submits-ind-application-for-an-igf-1r-antibody-for-the-treatment-of-thyroid-eye-disease/
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Lane, L. C., Cheetham, T. D., Perros, P., & Pearce, S. H. S. (2020). New therapeutic horizons for graves' hyperthyroidism. Endocrine Reviews, 41(6), 873-884. 022 [pii]
- ↑ Silkiss, R. Z., & Wade, A. R. (2016). Neuroanatomic variations in graves' dysthyroid ophthalmopathy as studied with MRI. Transactions of the American Ophthalmological Society, 114, T9. doi: T9
- ↑ Jump up to: 3.0 3.1 Silkiss, R. Z., Reier, A., Coleman, M., & Lauer, S. A. (2010). Rituximab for thyroid eye disease. Ophthalmic Plastic and Reconstructive Surgery, 26(5), 310-314 [doi]
- ↑ Jump up to: 4.0 4.1 4.2 Hegedüs, L., Smith, T. J., Douglas, R. S., & Nielsen, C. H. (2011). Targeted biological therapies for graves' disease and thyroid-associated ophthalmopathy. focus on B-cell depletion with rituximab. Clinical Endocrinology, 74(1), 1-8. doi:10.1111/j.1365-2265.2010. 03806.x [doi]
- ↑ Jump up to: 5.0 5.1 El Fassi, D., Clemmensen, O., Nielsen, C. H., Silkiss, R. Z., & Hegedüs, L. (2007). Evidence of intrathyroidal B-lymphocyte depletion after rituximab therapy in a patient with graves' disease. The Journal of Clinical Endocrinology and Metabolism, 92(10), 3762-3763. doi:92/10/3762 [pii]
- ↑ Jump up to: 6.0 6.1 Douglas, R. S., & Gupta, S. (2011). The pathophysiology of thyroid eye disease: Implications for immunotherapy. Current Opinion in Ophthalmology, 22(5), 385-390. doi:10.1097/ICU.0b013e3283499446 [doi]
- ↑ Salvi, M., Vannucchi, G., Currò, N., Campi, I., Covelli, D., Dazzi, D., . . . Beck-Peccoz, P. (2015). Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe graves' orbitopathy: A randomized controlled study. The Journal of Clinical Endocrinology and Metabolism, 100(2), 422-431. doi:10.1210/jc.2014-3014 [doi]
- ↑ Salvi, M., Vannucchi, G., Campi, I., & Beck-Peccoz, P. (2009). Rituximab in the treatment of thyroid eye disease: Science fiction? Orbit (Amsterdam, Netherlands), 28(4), 251-255. doi:10.1080/01676830903104611 [pii]
- ↑ Khanna, D., Chong, K. K., Afifiyan, N. F., Hwang, C. J., Lee, D. K., Garneau, H. C., . . . Douglas, R. S. (2010). Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology, 117(1), 133-139.e2. 2009.05.029 [doi]
- ↑ Salvi, M., Vannucchi, G., Currò, N., Introna, M., Rossi, S., Bonara, P., . . . Beck-Peccoz, P. (2012). Small dose of rituximab for graves orbitopathy: new insights into the mechanism of action. Archives of Ophthalmology (Chicago, Ill.: 1960), 130(1), 122-124. doi:10.1001/archopthalmol.2011.1215 [doi]
- ↑ Stan, M. N., Garrity, J. A., Carranza Leon, B. G., Prabin, T., Bradley, E. A., & Bahn, R. S. (2015). Randomized controlled trial of rituximab in patients with graves' orbitopathy. The Journal of Clinical Endocrinology and Metabolism, 100(2), 432-441. doi:10.1210/jc.2014-2572 [doi]
- ↑ Jump up to: 12.0 12.1 Li H, Yang L, Song Y, Zhao X, Sun C, Zhang L, Zhao H, Pan Y. Comparative effectiveness of different treatment modalities for active, moderate-to-severe Graves' orbitopathy: a systematic review and network meta-analysis. Acta Ophthalmol. 2021 Dec 16. doi: 10.1111/aos.15074. Epub ahead of print. PMID: 34918472.
- ↑ Strianese, D., & Rossi, F. (2019). Interruption of autoimmunity for thyroid eye disease: B-cell and T-cell strategy. Eye, 33(2), 191-199. doi:10.1038/s41433-018-0315-9
- ↑ Kahaly, G. J., Stan, M. N., Frommer, L., Gergely, P., Colin, L., Amer, A., . . . He, Y. (2020). A novel anti-CD40 monoclonal antibody, iscalimab, for control of graves hyperthyroidism-A proof-of-concept trial. The Journal of Clinical Endocrinology and Metabolism, 105(3), dgz013. doi: 10.1210/clinem/dgz013. 013 [pii]
- ↑ Roofeh, D., Lin, C. J. F., Goldin, J., Kim, G. H., Furst, D. E., Denton, C. P., . . . focuSSced Investigators. (2021). Tocilizumab prevents progression of early systemic sclerosis-associated interstitial lung disease. Arthritis & Rheumatology (Hoboken, N.J.), 73(7), 1301-1310. doi:10.1002/art.41668 [doi]
- ↑ Antonio, A. A., Santos, R. N., & Abariga, S. A. (2021). Tocilizumab for giant cell arteritis. The Cochrane Database of Systematic Reviews, 8(8), CD013484. doi: 10.1002/14651858.CD013484.pub2 [doi]
- ↑ Sarfraz, A., Sarfraz, Z., Sarfraz, M., Aftab, H., & Pervaiz, Z. (2021). Tocilizumab and COVID-19: A meta-analysis of 2120 patients with severe disease and implications for clinical trial methodologies. Turkish Journal of Medical Sciences, 51(3), 890-897. doi:10.3906/sag-2010-131 [doi]
- ↑ Perez-Moreiras, J. V., Gomez-Reino, J. J., Maneiro, J. R., Perez-Pampin, E., Romo Lopez, A., Rodríguez Alvarez, F. M., . . . Tocilizumab in Graves Orbitopathy Study Group. (2018). Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: A randomized clinical trial. American Journal of Ophthalmology, 195, 181-190. 0002-9394(18)30431-8 [pii]
- ↑ Sears CM, Azad AD, Topping KL, et al. (New Orleans, LA, 2022 November 2020). Recurrent disease and adverse events after tocilizumab treatment for thyroid eye disease. ASOPRS Fall Scientific Symposium.
- ↑ Sánchez-Bilbao, L., Martínez-López, D., Revenga, M., López-Vázquez, Á., Valls-Pascual, E., Atienza-Mateo, B., . . . Blanco, R. (2020). Anti-IL-6 receptor tocilizumab in refractory graves' orbitopathy: National multicenter observational study of 48 patients. Journal of Clinical Medicine, 9(9), 2816. doi: 10.3390/jcm9092816. [doi]
- ↑ Silkiss RZ, Paap MK, Roelofs KA, Agi J, Weis E. Treatment of corticosteroid-resistant thyroid eye disease with subcutaneous tocilizumab. Can J Ophthalmol. 2021 Feb;56(1):66-70. doi: 10.1016/j.jcjo.2020.07.020. Epub 2020 Sep
- ↑ Jump up to: 22.0 22.1 Tsui, S., Naik, V., Hoa, N., Hwang, C. J., Afifiyan, N. F., Sinha Hikim, A., . . . Smith, T. J. (2008). Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: A tale of two antigens implicated in graves' disease. Journal of Immunology (Baltimore, Md.: 1950), 181(6), 4397-4405. doi:181/6/4397 [pii]
- ↑ Pritchard, J., Han, R., Horst, N., Cruikshank, W. W., & Smith, T. J. (2003). Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with graves' disease is mediated through the insulin-like growth factor I receptor pathway. Journal of Immunology (Baltimore, Md.: 1950), 170(12), 6348-6354. doi:10.4049/jimmunol.170.12.6348 [doi]
- ↑ Chen, H., Mester, T., Raychaudhuri, N., Kauh, C. Y., Gupta, S., Smith, T. J., & Douglas, R. S. (2014). Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. The Journal of Clinical Endocrinology and Metabolism, 99(9), E1635-40. doi:10.1210/jc.2014-1580 [doi]
- ↑ Smith, T. J., Kahaly, G. J., Ezra, D. G., Fleming, J. C., Dailey, R. A., Tang, R. A., . . . Douglas, R. S. (2017). Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med, 376(18), 1748-1761. doi:10.1056/NEJMoa1614949
- ↑ Jump up to: 26.0 26.1 Douglas, R. S., Kahaly, G. J., Patel, A., Sile, S., Thompson, E. H. Z., Perdok, R., . . . Smith, T. J. (2020). Teprotumumab for the treatment of active thyroid eye disease. The New England Journal of Medicine, 382(4), 341-352. doi:10.1056/NEJMoa1910434 [doi]
- ↑ Hoang, T. D., Nguyen, N. T., Chou, E., & Shakir, M. K. (2021). Rapidly progressive cognitive decline associated with teprotumumab in thyroid eye disease. BMJ Case Reports, 14(5), e242153. doi: 10.1136/bcr-2021-242153. doi:10.1136/bcr-2021-242153 [doi]
- ↑ Sears CM, Pham B, Men C & Kossler AN. (November 2021). Teprotumumab for the Treatment of Recalcitrant TED. ASOPRS Fall Scientific Symposium.
- ↑ Douglas R.S., Francis-Sedlak M, Hold R.J. & Dailey R.A.& Kossler AN. (November 2021). Real-World Adherence with Teprotumumab in TED. ASOPRS Fall Scientific Symposium.
- ↑ Roopenian, D. C., & Akilesh, S. (2007). FcRn: The neonatal fc receptor comes of age. Nature Reviews Immunology, 7(9), 715-725. doi:10.1038/nri2155
- ↑ Robak, T., Kaźmierczak, M., Jarque, I., Musteata, V., Trelinski, J., Cooper, N., . . . Jolles, S. (2019). Rozanolixizumab, an anti-FcRn antibody: Final results from a phase II, multiple-dose study in patients with primary immune thrombocytopenia. Blood, 134, 897-897. doi:10.1182/blood-2019-129839
- ↑ Bril, V., Benatar, M., Andersen, H., Vissing, J., Brock, M., Greve, B., . . . MG0002 Investigators. (2021). Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: A phase 2 randomized control trial. Neurology, 96(6), e853-e865. doi:10.1212/WNL.0000000000011108 [doi]
- ↑ Ayabe, R., Rootman, D. B., Hwang, C. J., Ben-Artzi, A., & Goldberg, R. (2014). Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthalmic Plastic and Reconstructive Surgery, 30(5), 415-419. doi:10.1097/IOP.0000000000000211 [doi]
- ↑ Boskovic, O., Medenica, S., Radojevic, N., & Zarkovic, M. (2019). Etanercept in the treatment of graves' ophthalmopathy with primary hypothyroidism and rheumatoid arthritis. Central-European Journal of Immunology, 44(4), 463-465. doi:10.5114/ceji.2019.92803 [doi]
- ↑ Durrani, O. M., Reuser, T. Q., & Murray, P. I. (2005). Infliximab: A novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit (Amsterdam, Netherlands), 24(2), 117-119. 06QV378GJ21K05T [pii]
- ↑ Jansson, L., Vrolix, K., Jahraus, A., Martin, K. F., & Wraith, D. C. (2018). Immunotherapy with apitopes blocks the immune response to TSH receptor in HLA-DR transgenic mice. Endocrinology, 159(9), 3446-3457. doi:10.1210/en.2018-00306 [doi]