Anterior Chamber Paracentesis in Uveitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Description/Overview
Anterior, intermediate, and posterior uveitis, as well as panuveitis, contribute to more than 10% of visual impairment in the Western world.[1] Among these, anterior uveitis is the most common subtype, accounting for up to 60% of uveitis cases based on the International Uveitis Study Group classification.[2] Uveitis can arise from both noninfectious and infectious causes, with infectious uveitis representing approximately 10-20% of all uveitis cases.[3] Delayed or incorrect diagnoses can lead to visual impairment, undesirable side effects from inappropriate drug use, and uveitis-related complications.[4]
The diagnostic approach to uveitis primarily relies on a thorough medical history and clinical examination. However, sometimes the diagnosis is not straightforward, and it is not possible to determine the underlying cause. Laboratory screening, imaging, and invasive sampling techniques are employed in complex diagnostic cases.[5] Anterior chamber paracentesis, an invasive procedure that involves sampling the aqueous humor, can be used to aid in the diagnosis of uveitis.[6][7] This technique has been more widely performed in ophthalmology due to its relatively low risk of complications and minimal trauma to the eye compared to vitreous sampling. Moreover, it can be done quickly.
Performing definitive pathogen-specific PCR at an early stage can facilitate a timely diagnosis and initiation of appropriate therapy.
Diagnosis Yield of Anterior Chamber Paracentesis
Polymerase Chain Reaction (PCR)
Aqueous samples obtained through paracentesis can be used for culture when an infectious cause is suspected, although newer techniques for aqueous humor analysis, such as polymerase chain reaction (PCR), are more sensitive and are, therefore, prioritized over culture.[7] PCR is a fast and reliable method for identifying common pathogens causing uveitis. It can accurately detect small quantities of pathogenic DNA or RNA in the aqueous humor, making it particularly useful for diagnosis.[8][9][10][11]
Aqueous humor PCR testing specific to certain pathogens, such as herpes simplex virus, varicella-zoster virus, cytomegalovirus, and toxoplasmosis, has high diagnostic sensitivity and specificity comparable to vitreous biopsy and serologic tests.[12][13]
Aqueous PCR testing can change the diagnosis and treatment in a significant proportion of patients and is relatively safe.[14] It should be considered in uveitis cases with:
- Atypical clinical appearance;
- Recurrent uveitis of uncertain etiology;
- Therapy-refractory cases;
- Immunocompromised or older patients with misleading clinical presentations;
- Limited fundoscopic exam.
PCR from ocular fluids has a high reliability with low false-positive rates, although false-negative results can be challenging to verify due to the lack of a gold standard.[15]
PCR has certain drawbacks, including financial implications, restrictions on testing for multiple entities due to limited sample volume, the potential for amplifying contaminants erroneously, and the possibility of unsuccessful identification when cellular material is scarce.[12]
Overall, PCR, especially when applied to aqueous humor samples, shows good predictive and diagnostic value for anterior uveitis and panuveitis.[16]Its use can lead to early and accurate identification of infectious etiologies, guiding appropriate therapeutic interventions and improving patient outcomes.
Cytokine Biomarkers
Additionally, cytokine biomarkers can assist in the identification of uveitis etiologies. Ocular fluid samples from viral uveitis patients may show the presence of immune-regulatory cytokines such as IL-6, IL-10, and interferon-gamma (IFN-γ).[17] The measurement of these elevated cytokine levels can aid in distinguishing viral uveitis from other forms of the condition.
In the case of idiopathic uveitis, proinflammatory cytokines such as IL-1, IL-2, IL-6, TNF alpha (TNF-α), IFN-γ, IL-8, and monocyte chemotactic protein (MCP) 1 were found to be elevated in idiopathic uveitis.[18]
In cases of intermediate and posterior uveitis, cytokine analysis through an anterior chamber tap can be performed to assess IL-10 and -6 levels, along with cytology.[19] Cytokine analysis of vitreous and aqueous demonstrated predominantly higher levels of IL‑10 in active B‑cell lymphomas and elevated IL‑6 levels in uveitis. High levels of IL-10 and an IL-10/IL-6 ratio greater than 1 are indicative of primary intraocular lymphoma.[20] However, multiple reports have also demonstrated exceptions where elevated levels of IL-10 were noted in infectious uveitis and lower IL-10 levels were observed in early or low-grade vitreoretinal lymphoma.[21][22]
Among the diagnostic tests available, MyD88 L265P mutation analysis is a valuable tool for diagnosing B cell lymphoma.[23] Typically is performed on a sample obtained from the vitreous but, more recently, the analysis has also been performed on aqueous samples. It provides information on B cell clonality, supports the exclusion of uveitis, and may assist in the detection of recurrence and treatment monitoring.[24]
In summary, cytokine biomarkers obtained from anterior chamber paracentesis may potentially aid in the diagnosis and management of uveitis patients. However, there is no standard of criteria, and they are only suggestive and helpful in differentiating from other forms of uveitis.
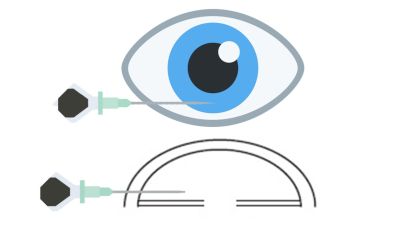
Technique/Procedure
AC paracentesis is a procedure performed in the outpatient clinic using a sterile technique.[25] Various methods have been described for its execution.[26] The technique can be employed with a slit lamp or without one, particularly in cases where patients are uncooperative or unable to sit or inexperienced physicians. However, when performed with the patient in the supine position, the risk of lens injury is minimized. Before the procedure, informed consent must be obtained, including a discussion of potential complications.
The steps involved in AC paracentesis are as follows:[27]
- Instillation of topical anesthetic drops.
- Application of topical povidone-iodine solution.
- Placement of a lid speculum to enhance visualization.
- Introduction of a 30-gauge needle attached to a 1 mL tuberculin syringe. The needle is advanced into the anterior chamber through the temporal limbus or clear cornea, parallel to the iris plane, with the tip positioned over the mid-periphery of the iris.
- Aspiration of approximately 0.1–0.2 mL of aqueous fluid, ensuring avoidance of contact with the iris and lens.
- Placement of a sterile cotton-tipped applicator at the entry point, followed by the application of gentle pressure for 10–20 seconds.
- Application of antibiotic eye drops and patching of the eye.
- Reassessment after approximately half an hour to confirm the reformation of the anterior chamber and exclude the presence of hyphema.
Source: YouTube. Retina and cataract Musha M.Anterior chamber or aqueous tap in phakic eye. https://www.youtube.com/watch?v=aPGwFkhCWGM Accessed May 22, 2023.
Complications
AC paracentesis, while generally safe, carries potential complications.[28]
- Amsler sign (vessels within the anterior chamber have a propensity to hemorrhage during this procedure);[7]
- Endophthalmitis;
- Corneal abscess;
- Conversion to vitreous sampling;
- Trauma to the cornea, iris, lens, and posterior segment;
- Corneal infections.
Conclusions
Anterior chamber paracentesis is an important diagnostic tool in uveitis management, mostly helping with the identification of infectious causes and guiding appropriate treatment strategies. Its use, in conjunction with PCR testing and/or cytokine biomarkers, offers a reliable and efficient approach to improve diagnostic accuracy and optimize therapeutic interventions, ultimately benefiting patients with uveitis.
Additional Resources
- Jabs, DA, McCluskey, P, Palestine, AG, Thorne, JE, The Standardization of Uveitis Nomenclature (SUN) Working Group, The standardisation of uveitis nomenclature (SUN) project. Clin Experiment Ophthalmol. 2022; 50( 9): 991- 1000. doi:10.1111/ceo.14175
- AAO: "When and How to Do an Anterior Chamber Tap", Dr. Debra Goldstein
References
- ↑ Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5-6):303-308. doi:10.1007/BF00163549
- ↑ Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group Recommendations for the Evaluation of Intraocular Inflammatory Disease. Am J Ophthalmol. 1987;103(2):234-235. doi:10.1016/S0002-9394(14)74235-7
- ↑ Tran THC. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. British Journal of Ophthalmology. 2003;87(1):79-83. doi:10.1136/bjo.87.1.79
- ↑ Choi W, Kang HG, Choi EY, et al. Clinical utility of aqueous humor polymerase chain reaction and serologic testing for suspected infectious uveitis: a single-center retrospective study in South Korea. BMC Ophthalmol. 2020;20(1):242. doi:10.1186/s12886-020-01513-x
- ↑ Hunter RS, Lobo AM. Current Diagnostic Approaches to Infectious Anterior Uveitis. Int Ophthalmol Clin. 2011;51(4):145-156. doi:10.1097/IIO.0b013e31822d6807
- ↑ Salmon J, Bowling B. Kanski’s Clinical Ophthalmology A Systematic Approach. Ninth. Elsevier; 2020.
- ↑ Jump up to: 7.0 7.1 7.2 Yanoff M, Duker JS. Ophthalmology. Fifth. Elsevier Inc.; 2019.
- ↑ Doan T, Acharya NR, Pinsky BA, et al. Metagenomic DNA Sequencing for the Diagnosis of Intraocular Infections. Ophthalmology. 2017;124(8):1247-1248. doi:10.1016/j.ophtha.2017.03.045
- ↑ Rothova A, de Boer JH, ten Dam-van Loon NH, et al. Usefulness of Aqueous Humor Analysis for the Diagnosis of Posterior Uveitis. Ophthalmology. 2008;115(2):306-311. doi:10.1016/j.ophtha.2007.05.014
- ↑ Sowmya P, Madhavan HN. Diagnostic Utility of Polymerase Chain Reaction on Intraocular Specimens to Establish the Etiology of Infectious Endophthalmitis. Eur J Ophthalmol. 2009;19(5):812-817. doi:10.1177/112067210901900520
- ↑ Taravati P, Lam D, Van Gelder RN. Role of Molecular Diagnostics in Ocular Microbiology. Curr Ophthalmol Rep. 2013;1(4):181-189. doi:10.1007/s40135-013-0025-1
- ↑ Jump up to: 12.0 12.1 Sen HN, Albini TA, Burkholder BM, et al. 2022-2023 Basic and Clinical Science Course, Section 9: Uveitis and Ocular Inflammation. (American Academy of Ophthalmology, ed.).; 2022.
- ↑ Putera I, La Distia Nora R, Utami N, et al. The impact of aqueous humor polymerase chain reaction and serological test results for establishing infectious uveitis diagnosis: An Indonesian experience. Heliyon. 2022;8(10). doi:10.1016/j.heliyon.2022.e10988
- ↑ Chronopoulos A, Roquelaure D, Souteyrand G, Seebach JD, Schutz JS, Thumann G. Aqueous humor polymerase chain reaction in uveitis - utility and safety. BMC Ophthalmol. 2016;16(1):1-7. doi:10.1186/s12886-016-0369-z
- ↑ de BOER JH, VERHAGEN C, BRUINENBERG M, et al. Serologic and Polymerase Chain Reaction Analysis of Intraocular Fluids in the Diagnosis of Infectious Uveitis. Am J Ophthalmol. 1996;121(6):650-658. doi:10.1016/S0002-9394(14)70631-2
- ↑ Choi W, Kang HG, Choi EY, et al. Clinical utility of aqueous humor polymerase chain reaction and serologic testing for suspected infectious uveitis: A single-center retrospective study in South Korea. BMC Ophthalmol. 2020;20(1). doi:10.1186/s12886-020-01513-x
- ↑ Balamurugan S, Das D, Hasanreisoglu M, et al. Interleukins and cytokine biomarkers in uveitis. Indian J Ophthalmol. 2020;68(9):1750. doi:10.4103/ijo.IJO_564_20
- ↑ Curnow SJ, Falciani F, Durrani OM, et al. Multiplex Bead Immunoassay Analysis of Aqueous Humor Reveals Distinct Cytokine Profiles In Uveitis. Investigative Opthalmology & Visual Science. 2005;46(11):4251. doi:10.1167/iovs.05-0444
- ↑ Sève P, Cacoub P, Bodaghi B, et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun Rev. 2017;16(12):1254-1264. doi:10.1016/j.autrev.2017.10.010
- ↑ Cassoux N, Giron A, Bodaghi B, et al. IL-10 Measurement in Aqueous Humor for Screening Patients with Suspicion of Primary Intraocular Lymphoma. Investigative Opthalmology & Visual Science. 2007;48(7):3253. doi:10.1167/iovs.06-0031
- ↑ Buggage RR. Primary Intraocular Lymphoma With a Low Interleukin 10 to Interleukin 6 Ratio and Heterogeneous IgH Gene Rearrangement. Archives of Ophthalmology. 1999;117(9):1239. doi:10.1001/archopht.117.9.1239
- ↑ Ongkosuwito J V, Feron EJ, van Doornik CE, et al. Analysis of immunoregulatory cytokines in ocular fluid samples from patients with uveitis. Invest Ophthalmol Vis Sci. 1998;39(13):2659-2665.
- ↑ Sehgal A, Pulido JS, Mashayekhi A, Milman T, Deák GG. Diagnosing Vitreoretinal Lymphomas—An Analysis of the Sensitivity of Existing Tools. Cancers (Basel). 2022;14(3):598. doi:10.3390/cancers14030598
- ↑ Hiemcke-Jiwa LS, ten Dam-van Loon NH, Leguit RJ, et al. Potential Diagnosis of Vitreoretinal Lymphoma by Detection of MYD88 Mutation in Aqueous Humor With Ultrasensitive Droplet Digital Polymerase Chain Reaction. JAMA Ophthalmol. 2018;136(10):1098. doi:10.1001/jamaophthalmol.2018.2887
- ↑ Trivedi D, Denniston AK, Murray PI. Safety profile of anterior chamber paracentesis performed at the slit lamp. Clin Exp Ophthalmol. 2011;39(8):725-728. doi:10.1111/j.1442-9071.2011.02565.x
- ↑ Van der Lelij A, Rothova A. Diagnostic anterior chamber paracentesis in uveitis: a safe procedure? British Journal of Ophthalmology. 1997;81(11):976-979. doi:10.1136/bjo.81.11.976
- ↑ Blomquist PH, American Academy of Ophthalmology. Practical Ophthalmology : Manual for Beginning Residents.
- ↑ Kitazawa K, Sotozono C, Koizumi N, et al. Safety of anterior chamber paracentesis using a 30-gauge needle integrated with a specially designed disposable pipette. British Journal of Ophthalmology. 2017;101(5):548-550. doi:10.1136/bjophthalmol-2016-309650