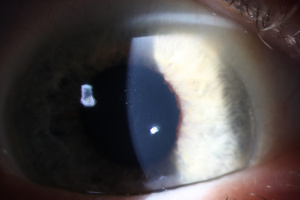
Amantadine Induced Corneal Edema
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
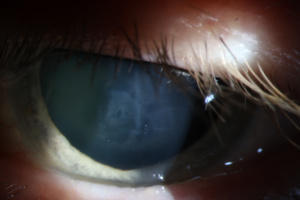
Originally developed as a prophylactic treatment for influenza A, amantadine is now used for treatment of tremors associated with Parkinson’s disease (PD), extrapyramidal side effects of other antipsychotic medications, and fatigue in patients with multiple sclerosis. There are known side effects of amantadine, such as neuroleptic malignant syndrome, light-headedness and drowsiness, orthostatic hypotension, dry mouth, constipation, Stevens-Johnson syndrome, and skin rashes. A rare side effect of amantadine toxicity is bilateral corneal edema.
Etiology
Ocular toxicity from amantadine use is extremely rare, with a diagnosis of corneal edema or Fuchs dystrophy in 0.27% of patients during a two-year surveillance study performed at the Veteran’s Health Administration [1]. A separate 2016 study looked at data from the Taiwan Longitudinal Insurance Database and found the relative risk (RR) of corneal edema in patients with Parkinson’s disease on amantadine to be 1.98 [2].
Other dopaminergic agonists have been shown to cause similar corneal edema, which reversed after the agent was stopped. These include methylphenidate [3], ropinirole [3], and bupropion [4].
Risk Factors
Known risk factors of amantadine toxicity include higher daily dose and cumulative dose. In addition, patients on multiple dopaminergic agonists may be at increased risk of developing corneal edema due to an additive effect.
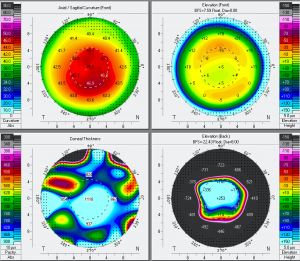
Three commonly used metrics to evaluate corneal endothelial health include endothelial cell density (ECD), percentage of endothelial hexagonal cells (EH), and coefficient of variation (CoV). A 2010 cross-sectional study found that Parkinson’s patients taking amantadine had lower ECD, EH, and higher CoV than age-matched controls [5]. Furthermore, there was a trend toward lower ECD and EH, and increased CoV, among patients with a higher cumulative lifetime dose of amantadine. Another study demonstrated that when stratifying patients taking amantadine into subgroups based on their daily dose, patients taking >100 mg/day had a RR of 2.71, while patients on a daily dose of <100 mg/day had a RR of 1.69 [2] of developing corneal edema [2].
A 2019 prospective study evaluated the corneal endothelium health among 90 patients with Parkinson’s Disease on amantadine, 30 patients with Parkinson’s disease who have never taken amantadine, and 30 healthy controls over the course of one year and found that patients taking amantadine had a larger decrease in ECD, EH, and a larger increase in CoV than amantadine naïve Parkinson’s disease patients or healthy controls. When stratified by daily dose of amantadine (200 mg, 300 mg, or 400 mg), higher doses were associated with a larger decrease in EH and a larger increase in CoV; however, differences in ECD did not meet the threshold for statistical significance, but followed the same trend [6].
Pathophysiology
While the exact mechanism remains unclear, several studies have demonstrated that amantadine has dose-dependent deleterious effects upon the corneal endothelium, even in the absence of clinically evident changes.
Two case reports detail patients in whom keratoplasty was required after developing amantadine-induced corneal edema [7] [8]. In both cases, histopathology showed moderate to complete loss of the corneal endothelium without guttata or inflammation. One possible explanation is that there may be medication-induced stress on the cornea endothelium, which at a threshold level can lead to endothelial cell dysfunction/loss and subsequently clinically significant corneal edema.
Diagnosis
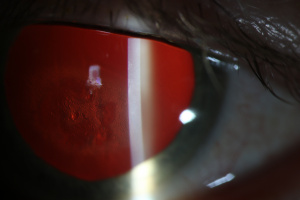
Diagnosis of amantadine-induced corneal edema is clinical. Incidence of edema is highest in the first weeks and months after starting the medication, but has been reported several years after initiating therapy [1][9]. Key exam findings include a lack of anterior chamber inflammation, deep stromal vessels, or endothelial guttata; presence of one or more of these clinical signs would suggest a different etiology (see below).
Corneal sensation is typically intact and presence of intact cornea sensation helps to distinguish this medication-induced toxicity from a viral etiology (HSV, VZV). Endothelial cell count may be useful to confirm a decrease in endothelial cell density.
Differential diagnosis
- Fuchs’ Endothelial Dystrophy
- Posterior Polymorphous Corneal Dystrophy (PPMD)
- Iridocorneal Endothelial (ICE) Syndrome
- HSV Keratitis
- Interstitial Keratitis
- Pseudophakic/Aphakic Bullous Keratopathy
Management
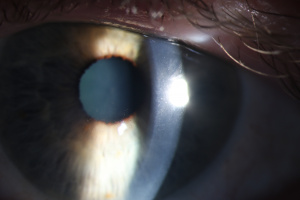
In most reported cases, corneal edema resolves within weeks after discontinuing amantadine therapy; however, early diagnosis is crucial as prolonged corneal edema in the setting of toxicity can be irreversible [10]. Keratoplasty is rarely required for resolution of the edema; however, the corneal edema has been reported to recur following keratoplasty if amantadine use is not discontinued [7][8].
Prognosis
Most patients have a good visual outcome with reversal of corneal edema if amantadine use is promptly stopped. However, a 2023 published case report[11] presented a patient who had been taking amantadine for ten years and at 3 months after discontinuing her amantadine therapy, still did not have resolution of her corneal edema. The authors documented a significant reduction in endothelial cells and they suggested that in patients taking amantadine, monitoring for early signs of endothelial cell loss should be considered.
References
- ↑ Jump up to: 1.0 1.1 French DD, Margo CE. Postmarketing surveillance of corneal edema, Fuchs dystrophy, and amantadine use in the Veterans Health Administration. Cornea. 2007;26(9):1087-1089.
- ↑ Jump up to: 2.0 2.1 2.2 Lee PY, Tu HP, Lin CP, et al. Amantadine use as a risk factor for corneal edema: a nationwide cohort study in Taiwan. Am J Ophthalmol. 2016;171:122-129.
- ↑ Jump up to: 3.0 3.1 Mancera N, Wadia HP. Corneal edema associated with systemic dopaminergic agents. Cornea. 2019;38(8):1040-1042.
- ↑ Yang Y, Teja S, Baig K. Bilateral corneal edema associated with amantadine. CMAJ. 2015;187(15):1155-1158.
- ↑ Chang KC, Jeong JH, Kim MK, Wee WR, Lee JH, Jeon BS. The effect of amantadine on corneal endothelium in subjects with Parkinson’s disease. Ophthalmology. 2010;117(6):1214-1219.
- ↑ Daggumilli S, Vanathi M, Ganger A, Goyal V, Tandon R. Corneal evaluation in patients with parkinsonism on long-term amantadine therapy. Cornea. 2019;38(9):1131-1136.
- ↑ Jump up to: 7.0 7.1 Welder J, Kardon RH, Cohen A, Wagoner MD, Bilateral Corneal Edema Associated with Amantadine Use. Eyerounds.org. September 27, 2010; Available from: http://www.EyeRounds.org/cases/123-amantadine-corneal-edema.htm.
- ↑ Jump up to: 8.0 8.1 Koenig SB, McDermott ML, Simons KB. Nonimmunologic graft failure after descemet’s stripping automated endothelial keratoplasty (Dsaek) for presumed amantadine-induced corneal edema. Eye & Contact Lens. 2009;35(4):209–211.
- ↑ Chang KC, Kim MK, Wee WR, Lee JH. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea. 2008;27(10):1182–1185.
- ↑ Jeng BH, Galor A, Lee MS, et al. Amantadine-associated corneal edema. Ophthalmology 2008;115:1540-1544.
- ↑ Raharja, Mina, Ashena, "Amantadine-induced corneal edema: A case and literature review," AJO Case Reports, available online 3 July 2023, 101881.