Fuchs’ Endothelial Dystrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Fuchs’ endothelial dystrophy is a non-inflammatory, sporadic or autosomal dominant, dystrophy involving the endothelial layer of the cornea. With Fuchs’ dystrophy the cornea begins to swell causing glare, halo, and reduced visual acuity. The damage to the cornea in Fuchs’ endothelial dystrophy can be so severe as to cause corneal blindness.
Disease Entity
Fuchs' Dystrophy ICD-9 371.57
Disease
Fuchs’ endothelial dystrophy is a non-inflammatory, sporadic or autosomal dominant, dystrophy involving the endothelial layer of the cornea. Over the course of decades, the cornea develops guttae and increases in thickness, causing glare, halos, and reduced visual acuity. The damage to the cornea in Fuchs’ endothelial dystrophy can be so severe as to cause corneal blindness.
Etiology
Fuchs’ dystrophy is often inherited in an autosomal dominant manner. This means if you have an inherited form of Fuchs’ endothelial dystrophy there is a 50% chance you will pass it on to your children. Spontaneous mutations in the genes for Fuchs’ dystrophy also can cause new Fuchs’ dystrophy in a person with no family history.
Genetics
The genetics of classic, late-onset Fuchs' dystrophy is complex and multifactorial. Among the various genetic variants associated with disease, the strongest association identified has been with expansion of the CTG18.1 trinucleotide repeat in TCF4.[1] In most people, the "CTG" set of three consecutive nucleotides at this specific location in their DNA repeats approximately 10 to 20 times, whereas in most people with Fuchs dystrophy, it repeats at least 40 to 50 times, averaging close to 100 repeats.[2] In large cohorts of people with Fuchs' dystrophy, approximately two out of three people harbor this genetic variant, an expanded trinucleotide repeat.[1][2]
In addition to the contribution of this major variant, the phenotype, or clinical presentation of disease, appears to be the result of multiple genetic inputs. For example, clinical signs may manifest more severely in some cases where both parents are affected[3] or if two separate genetic factors are present in an individual,[4] suggesting a role for interaction among genes. The strongest evidence for this phenomenon has arisen from a large genome-wide association study that pooled data from a number of teams studying Fuchs' dystrophy, each contributing genetic and clinical data from their own cohorts.[5] By collaborating together, this approach garnered the statistical power to discern that, while the TCF4 genetic variant demonstrated the strongest association, three additional chromosomal loci each significantly contributed to risk of disease, located at the KANK4, LAMC1 and LINC00970/ATP1B1 genes. Each variant was found to contribute to risk, and in the case of LAMC1 and TCF4 variants, the degree of risk also varied by sex.
Studies conducted among large families with Fuchs' dystrophy have identified additional genetic variants that segregate with the disease phenotype, meaning that family members affected by the disease carry a genetic variant that does not appear in family members without the disease. These are likely associated with a small proportion of Fuchs' dystrophy in the overall population, and includes variants in Transcription factor 8 (TCF8) (chr. 10)[6], ATP/GTP binding protein-like 1 (AGBL1) (chr. 15)[7], lipoxygenase homology domain 1 (LOXHD1) (chr. 18)[8], solute carrier family 4 member 11 (SLC4A11) (chr. 20) gene [9] and Transforming growth factor-β–induced and clusterin[10].
An early-onset form of Fuchs' dystrophy is caused by mutations in the COL8A2 gene[11] and is associated with formation of bullous keratopathy, or corneal blisters, within the first few decades of life.[12] Descemet membrane is markedly thickened and guttae appear buried rather than protruding posteriorly.[13] The pattern of inheritance of such mutations appears to be autosomal dominant with a relatively equal distribution between men and women.
Risk Factors
The most prevalent genetic risk factor for Fuchs’ dystrophy is the CTG18.1 trinucleotide repeat expansion in TCF4.[1] Affected individuals have at least a 50% chance of passing the gene on to their children.
Environmental risk factors include smoking and body mass index. In the Reykjavik Eye Study, participants with a 20-pack year history of smoking experienced more than double the risk of cornea guttata. Higher weight and body mass index were associated with decreased risk of cornea guttata. [14]
Fuchs’ dystrophy is rarely seen in people younger than 30 to 40 years of age and seems to present slightly earlier in women.
General Pathology
In the early stages of Fuchs’ dystrophy, loss of endothelial cells and small excrescences of Descemet membrane can be seen. These excrescences are called 'guttae' and look similar to microscopic mushroom caps on the endothelial surface of the cornea. These guttae are visible on slit lamp exam. The endothelial cells may appear larger than average and may have embedded pigment. With time, due to the compromised watertight seal normally provided by the endothelium, fluid from the anterior chamber will collect in the corneal stroma, increasing the thickness of the corneal stroma and causing scattering of light. With more advanced disease, the swelling, or edema, collects in the epithelial layer of the cornea, causing small blisters called bullae. With chronic edema, fibrotic tissue will form in the subepithelial space and invade the cornea leading to further corneal opacification. Permanent scar tissue can eventually develop in the cornea.
The American Academy of Ophthalmology's Pathology Atlas contains a virtual microscopy image of Fuchs' Endothelial Dystrophy.
Pathophysiology
The stroma of the cornea is composed of 78% water. The endothelial cells of the cornea are responsible for maintaining the delicate hydration status (78% water) of the corneal stroma. Although in early Fuchs’ dystrophy there are enough healthy endothelial cells to prevent the cornea from becoming too saturated with extra water, eventually enough cells are damaged that those remaining cannot keep up with the osmotic pressure. At this point, fluid begins to collect in the corneal stroma which can result in blurry vision. The excess fluid will eventually migrate to the corneal epithelium causing bullae, which may break and cause pain and/or create a risk for infection within open surface wounds. These changes lead to chronic irritation and inflammation causing scar tissue and possible pannus formation.
Primary Prevention
Fuchs’ dystrophy is an inherited corneal dystrophy affecting the endothelium. There is no primary prevention for this disease entity.
Diagnosis
The diagnosis of Fuchs' Endothelial Dystrophy is primary clinical, based on history and slit lamp exam of the eye.
History
The classic history for Fuchs’ endothelial dystrophy is a patient, more commonly a woman, in the fourth to fifth decade of life, with symptoms of reduced or fluctuating vision, glare, or in some cases recurrent foreign body sensation. Patients classically experience morning blurry vision, as evaporation at the corneal surface is unable to occur overnight with eyelids closed, and the cornea swells during sleep. Patients may have a family history of a corneal transplantation in one or more family members.
Physical Examination
Slit lamp exam will vary depending on the severity of the dystrophy.
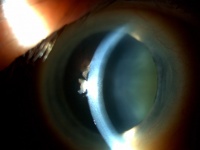
In very mild dystrophy, guttae can be seen on the corneal endothelium.
The guttae are often more marked in the central cornea and will be bilateral, though one eye may be more severe than the other.
In more advanced Fuchs’ dystrophy a haze will develop in the corneal stroma. As the stroma thickens, folds in the Descemet’s membrane and endothelium will be visible. As additional endothelial cells are lost, the corneal edema worsens and fluid collects in the epithelium forming microcystic changes as well as large epithelial bullae . Microcysts are best seen after fluorescein is placed on the cornea leaving areas of negative staining. If a bulla has recently ruptured, an epithelial defect may be seen. In more severe, long standing, cases of Fuchs’ dystrophy, dense corneal edema and bullous keratopathy are seen. The corneal opacification can be severe enough to prevent visualization of the anterior segment structures as well as the endothelium. The chronic corneal edema will induce sub-epithelial fibrosis as well as corneal vascularization.
Signs
The most common signs of Fuchs' Dystrophy include:
- Guttata on Descemet's membrane: the guttata tend to be central and slowly become more prominent peripherally
- Stromal edema
- Endothelial folds
- Epithelial microcysts
- Epithelial bullae
- Sub-epithelial fibrosis
- Stromal haze and scar formation
- Vascular ingrowth into the cornea
Symptoms
Some of the earliest symptoms of Fuchs’ endothelial dystrophy include reduced contrast sensitivity and mild reduction of visual acuity. Patients will often notice glare around a point source of light or have difficulty with nighttime driving. As the dystrophy is often slow in onset, patients may not even notice that their vision is reduced. Once fluid begins to collect in the stroma, patients will start to notice fluctuation in vision, typically worse in the early AM and improving toward the end of the day. Vision may be worse on humid or rainy days, and better on dry days. Eventually the vision stops fluctuating and becomes constantly blurry. Slowly, the vision continues to worsen, and eventually intermittent pain can be felt if bullae form and rupture leaving corneal epithelial defects.
Clinical diagnosis
The diagnosis of Fuchs’ endothelial dystrophy is clinical. The diagnosis is relatively easy in early disease as you can see the endothelial changes as well as mild corneal stromal edema. In severe cases, where you cannot see the endothelium, the diagnosis can be more challenging, and the diagnosis may need to be based on the contralateral eye or by history.
Diagnostic procedures
The diagnosis of Fuchs’ endothelial dystrophy is clinical; however, there are some diagnostic tests that can be helpful. Pachymetry, or measurement of the central corneal thickness, is helpful in following a patient with Fuchs’ dystrophy. Over time you will see increasing corneal thickness as the disease worsens. The rate at which you see increasing corneal thickness can help with counseling patients. The corneal thickness also may help with risk/benefit analysis of any other surgery that may be necessary (such as cataract surgery). Endothelial cell counts can also be helpful when counseling patients as to how quickly their dystrophy may progress as well as how safe any other intra-ocular surgery may be. In even moderate Fuchs’ dystrophy, the cell count can be very difficult to obtain. Evaluation of the endothelium by specular microscopy can demonstrate classic changes of Fuchs' endothelial dystrophy, including guttae, variation in cell size and shape, and low cell count per unit area.
Laboratory test
Fuchs’ endothelial dystrophy is diagnosed clinically. Specular microscopy to visualize the endothelium can corroborate the typical endothelial changes associated with this dystrophy.
Differential diagnosis
The differential diagnosis for Fuchs’ endothelial dystrophy includes anything that could induce endothelial deposits and/or corneal swelling:
- Pigment dispersion syndrome
- Keratic precipitates from uveitis
- Herpetic stromal keratitis
- Pseudophakic or aphakic bullous keratopathy
- Iridocorneal endothelial (ICE) dystrophy
- Congenital hereditary endothelial dystrophy
- Congenital stromal dystrophy
- Toxic anterior segment syndrome
- Posterior Polymorphic Membrane Dystrophy
Management
Medical therapy
Medical treatment of Fuchs’ dystrophy begins once patients notice fluctuations in vision. The early treatment is usually in the form of hypertonic saline (such as Muro 128 or sodium chloride) eye drops and/or ointments. Use of the hypertonic saline may stabilize or improve vision by drawing extra water out of the cornea. Any activity that helps to evaporate fluid off the cornea will help shorten the time to visual recovery. Such activities may include pointing car vents toward the face or blowing air by the eyes using a hair dryer at arm's length. Bandage contact lenses can also be quite helpful in management of painful ruptured bullae in more severe disease.
Medical follow up
Patients with Fuchs’ dystrophy should be followed depending on the severity of disease. Patients with only mild guttata and minimal to no corneal stromal edema may be followed every 6-12 months. Patients with more severe disease, on maximal medical treatment, might be followed more closely to make sure treatment is adequate. Any patient using a bandage contact lens needs very close follow-up due to the risk of infection.
Surgery
As Fuchs’ dystrophy progresses, medical treatment may fail, and surgical management becomes necessary.
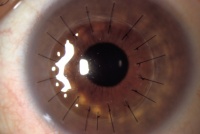
Penetrating keratoplasty (PKP or PK):
For many years, the only option for patients with visually significant Fuchs’ dystrophy was a full thickness corneal transplant or penetrating keratoplasty (PKP). A corneal transplant involves replacement of the full thickness of the cornea. The cornea is held in place with multiple sutures (as shown in the diagram on the right with 17 sutures) and some sutures may stay in place for several months or even years. Though the surgery can be successful, recovery can be relatively slow, sometimes taking a year or more for full visual recovery. It may take a year or longer for vision to stabilize and strong glasses or specialty fit contact lenses are often required to achieve the best vision after surgery. Patients may need to administer steroid eye drops (and be monitored while on these drops) for many years to prevent rejection of the cornea graft, steroid induced glaucoma, infections. The advantage of a full thickness corneal transplant is that it can restore vision even in the most advanced stages of Fuchs’ dystrophy.
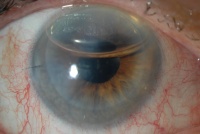
Descemet's Stripping Endothelial Keratoplasty (DSEK):
Since the early 2000s, there has been a trend to try and treat endothelial dystrophies by transplanting only the posterior, or endothelial, portion of the cornea. Posterior lamellar surgery (also referred to as endothelial keratoplasty) is now the standard of care in treatment of early to moderate Fuchs’ endothelial dystrophy.
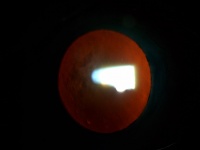
In Descemet Stripping Automated Endothelial Keratoplasty (DSEK), only Descemet membrane and the endothelial layer are removed from the affected cornea and replaced by a thin strip of donor posterior corneal stroma with attached Descemet's membrane and healthy endothelial cells. This surgery can be performed through a much smaller incision than traditional penetrating keratoplasty. Once the donor tissue is implanted into the eye, it is temporarily held in place by an air bubble (photo below). The air bubble usually dissolves over a couple of days. For the first day, the patient must remain in the supine (face up) position in order to keep the bubble centered and help the graft attach. (Exact instructions regarding position vary from surgeon to surgeon.) The new, transplanted endothelial cells pump excess water out of the cornea and restore cornea clarity resulting in the restoration of better quality vision.
Using this technique, only a few (or sometimes no) sutures are required and suture-induced astigmatism is less significant than with PKP. This results in better quality postoperative vision without glasses. Although glasses are still often required after DSEK, the prescription tends to be much less strong compared to post-PKP prescriptions. DSEK patients also recover vision more quickly than PKP patients.
The smaller DSEK wound results in fewer wound leaks, less chance of postoperative infection, and greater stability of the eye. Compared to eyes with PKP, eyes with DSEK are more resistant to damage in the unfortunate event of ocular trauma.
As with full thickness corneal transplants, other necessary intra-ocular surgery (such as cataract surgery) can sometimes be performed at the same time as DSEK.
Endothelial keratoplasty is a better option for mild to moderate Fuchs’ dystrophy that PKP. Once the cornea is scarred from chronic swelling, an endothelial transplant may not give satisfactory visual results. In these more advanced Fuchs' cases, a full PKP may be necessary.
Please visit the eyewiki DSEK page (https://eyewiki.aao.org/Descemet_Stripping_Endothelial_Keratoplasty) for more information.
Descemet's Membrane Endothelial Keratoplasty (DMEK):
The next iteration of corneal transplant endothelial keratoplaty surgery is called DMEK. Developed in 2006 by Dr Gerrit Melles, this elegant procedure transplants an even thinner sliver of tissue consisting only of Descemet membrane and endothelial cells (without any corneal stroma). The procedure is similar to DSEK; however, a different surgical technique is used to handle this very delicate tissue and deliver it into the eye and place it in the correct position. Similar to DSEK, an air (or sometime gas) bubble is placed in the eye to help the transplanted tissue attach and the patient must remain in the supine (face up) position for 1-3 days (with breaks to decrease the risk of pupillary block) following surgery. (Exact positioning instructions vary from surgeon to surgeon.) Because the tissue is thinner than DSEK tissue, it can be more challenging to attach and the need for longer periods of supine positioning or possible "re-bubbling" procedures may be higher with DMEK compared to DSEK. However, the thinner tissue with DMEK also translates into better quality vision for many patients.
Please visit the eyewiki DMEK page (https://eyewiki.org/Descemet_Membrane_Endothelial_Keratoplasty) for more information.
Descemetorhexis Without Endothelial Keratoplasty (DWEK)
Also sometimes referred to as Descemet's Stripping Only (DSO), DWEK is the latest surgical option for some patients with Fuchs'. Select patients with guttae localized in the central cornea and with a fairly healthy peripheral endothelium are candidates for the procedure. During DWEK, the ophthalmologist carefully strips away a 4mm circle of central diseased endothelial cells without placing any donor cornea tissue. If the surgery succeeds, healthy endothelial cells from the intact periphery will migrate centrally, covering the defect, and providing good quality vision. The surgery can be abetted by prescribing a rho kinase inhibitor eye drop for the patient to use post-operatively. (Note that the use of this drop in this setting does not currently have FDA approval in the US.) The potential benefits of this surgery include a very small wound, no need for post-operative supine positioning of the patient, no concern for rejection of transplanted tissue (since no tissue is implanted), and no need for long term monitoring while on steroid drops (usually used for many months or years to prevent rejection of transplanted cornea tissue in PK, DSEK, and DMEK). One main drawback of the surgery is that the central cornea will swell up right after surgery and the vision will become temporarily worse. The other drawback is that DWEK is not successful in all cases and some patients will endure worse vision for awhile and then go on to require DMEK or DSEK.
Please visit the eyewiki DWEK page (https://eyewiki.aao.org/Descemetorhexis_Without_Endothelial_Keratoplasty) for more information.
Surgical follow up
Follow-up is essential after any form of corneal transplantation. Most patients will need to be seen often in the first few weeks after the surgery to ensure surgical success and monitor for infection. Routine follow-up visits are essential for evaluation of transplant health, wound healing, and visual recovery including removal of sutures minimizing astigmatism. PKP, DSEK, and DMEK transplants have the potential for rejection, just as with any organ transplant. Rejection of the corneal transplant can occur at any point after surgery. Follow-up is essential in order to prevent and treat rejection if seen. Follow-up is also necessary to monitor for glaucoma, a possible side effect of long-term steroid drops used to prevent rejection. Glaucoma can cause permanent vision loss in some transplant patients. In addition to signs and symptoms of elevated eye pressure, corneal transplant recipients must be well informed about the signs and symptoms of infection and retinal detachment, and understand the importance of when and how to seek urgent eye care.
Complications
Surgical complications include infection, bleeding, wound leaks, transplant rejection, and suture related complications. The risks are higher for PKs compared with one of the EKs. With a full thickness transplant high refractive error and astigmatism can also be a problem. Long term use of topical steroids, necessary to prevent rejection, can induce cataract and glaucoma. DSEK and DMEK surgeries have risks of detachment of the graft with the consequent need for re-bubbling and repeat supine positioning and, occasionally, exchange of a graft that isn't attaching for a new graft. There is a risk of pupillary block associated with the use of air or gas bubbles in DSEK and DMEK. Complications can be associated with pain or loss of vision and can lead to the need for additional procedures and office visits.
Prognosis
The prognosis for patients with Fuchs' endothelial dystrophy is excellent. The various surgical treatments available today have very good success rates.
Additional Resources
- http://www.cornealdystrophyfoundation.org
- Boyd K, Jimenez EM. Corneal Dystrophies. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/corneal-dystrophies-list. Accessed March 07, 2019.
- Boyd K, Yeu E. Fuchs’ Dystrophy. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/fuchs-dystrophy-list. Accessed March 07, 2019.
References
- External Disease and Cornea, Section 8. Basic and Clinical Science Course, AAO, 2006. Cornea Atlas, 2nd Edition. Krachmer, Palay. Elsevier, 2006.
- Ocular Pathology Atlas. American Academy of Ophthalmology Web site. https://www.aao.org/resident-course/pathology-atlas. Published 2016. Accessed January 4, 2017.
- Weiss JS, Møllwe HU, Lisch W et al. The IC3D Classification of the Corneal Dystrophies. Cornea. 2008; 27: S1-S42.
- ↑ 1.0 1.1 1.2 Wieben ED, Aleff RA, Tosakulwong N, Butz ML, Highsmith WE, et al. 2012. A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2–2) gene predicts Fuchs corneal dystrophy. PLOS ONE 7:e49083
- ↑ 2.0 2.1 Vasanth S, Eghrari AO, Gapsis BC, Wang J, Haller NF, Stark WJ, Katsanis N, Riazuddin SA, Gottsch JD. Expansion of CTG18.1 Trinucleotide Repeat in TCF4 Is a Potent Driver of Fuchs' Corneal Dystrophy. Invest Ophthalmol Vis Sci. 2015 Jul;56(8):4531-6. doi: 10.1167/iovs.14-16122. PMID: 26200491; PMCID: PMC4515948.
- ↑ Meadows DN, Eghrari AO, Riazuddin SA, Emmert DG, Katsanis N, Gottsch JD. Progression of Fuchs corneal dystrophy in a family linked to the FCD1 locus. Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5662-6. doi: 10.1167/iovs.09-3568. Epub 2009 Jul 15. PMID: 19608546.
- ↑ Riazuddin SA, Zaghloul NA, Al-Saif A, Davey L, Diplas BH, Meadows DN, Eghrari AO, Minear MA, Li YJ, Klintworth GK, Afshari N, Gregory SG, Gottsch JD, Katsanis N. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am J Hum Genet. 2010 Jan;86(1):45-53. doi: 10.1016/j.ajhg.2009.12.001. Epub 2009 Dec 31. PMID: 20036349; PMCID: PMC2801746.
- ↑ Afshari NA, Igo RP Jr, Morris NJ, Stambolian D, Sharma S, Pulagam VL, Dunn S, Stamler JF, Truitt BJ, Rimmler J, Kuot A, Croasdale CR, Qin X, Burdon KP, Riazuddin SA, Mills R, Klebe S, Minear MA, Zhao J, Balajonda E, Rosenwasser GO, Baratz KH, Mootha VV, Patel SV, Gregory SG, Bailey-Wilson JE, Price MO, Price FW Jr, Craig JE, Fingert JH, Gottsch JD, Aldave AJ, Klintworth GK, Lass JH, Li YJ, Iyengar SK. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nat Commun. 2017 Mar 30;8:14898. doi: 10.1038/ncomms14898. PMID: 28358029; PMCID: PMC5379100.
- ↑ Mehta JS, Vithana EN, Tan DT, Yong VH, Yam GH, et al. 2008. Analysis of the posterior polymorphous corneal dystrophy 3 gene, TCF8, in late-onset Fuchs endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 49:184–88
- ↑ Riazuddin SA, Vasanth S, Katsanis N, Gottsch JD. 2013. Mutations in AGBL1 cause dominant late-onset Fuchs corneal dystrophy and alter protein-protein interaction with TCF4. Am. J. Hum. Genet. 93:758–64
- ↑ Riazuddin SA, Parker DS, McGlumphy EJ, Oh EC, Iliff BW, et al. 2012. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am. J. Hum. Genet. 90:533–39
- ↑ Loganathan SK, Casey JR. 2014. Corneal dystrophy-causing SLC4A11 mutants: suitability for folding-correction therapy. Hum. Mutat. 35:1082–91
- ↑ Jurkunas UV, Bitar M, Rawe I. 2009. Colocalization of increased transforming growth factor-beta-induced protein (TGFBIp) and Clusterin in Fuchs endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 50:1129–36
- ↑ Gottsch JD, Sundin OH, Liu SH, Jun AS, Broman KW, et al. 2005a. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of Fuchs corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 46:1934–39
- ↑ Eghrari AO, Riazuddin SA, Gottsch JD. Distinct Clinical Phenotype of Corneal Dystrophy Predicts the p.(Leu450Trp) Substitution in COL8A2. Cornea. 2016 May;35(5):587-91. doi: 10.1097/ICO.0000000000000796. PMID: 26989952.
- ↑ Gottsch JD, Zhang C, Sundin OH, Bell WR, Stark WJ, Green WR. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005 Dec;46(12):4504-11. doi: 10.1167/iovs.05-0497. PMID: 16303941.
- ↑ Zoega GM, Fujisawa A, Sasaki H, Kubota A, Sasaki K, Kitagawa K, Jonasson F. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology. 2006 Apr;113(4):565-9. doi: 10.1016/j.ophtha.2005.12.014. PMID: 16581419.