Acute Disseminated Encephalomyelitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Acute disseminated encephalomyelitis (ADEM) is an acute, autoimmune, demyelinating disease that causes inflammation of the brain and spinal cord, predominantly in children. It is caused by immune-mediated damage to the myelin sheath of the CNS nerves and typically presents clinically with new-onset multifocal neurologic deficits. Neuroimaging reveals areas of demyelination seen as T2 hyperintense white matter lesions with or without contrast enhancement. The disease often presents similarly to multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD), which are in the differential diagnosis of CNS demyelinating disease, however, ADEM is frequently monophasic and more associated to myelin oligodendrocyte antibodies (MOG-Ab)[1].
Epidemiology
The incidence of childhood ADEM has been reported as 0.23 to 0.4 per 100,000 patients.[2] The disease affects children (especially those under 10) preferentially to adults. The mean age of onset of ADEM is between 3.6 to 7 years of age. [2][3][4][5][6] Male children tend to be affected more frequently than female children.[3][4] Similar to MS, ADEM has demonstrated a geographical epidemiologic pattern, with one study showing an increased number of cases in the Northwest USA compared to the Southern USA (0.38 vs. 0.22 cases per 100,000, respectively).[7]There is also evidence that prevalence of ADEM increases with distance from the equator.[2]
Etiology
Before the vaccination era, ADEM was frequently associated with measles [8]. Today, ADEM is more commonly associated with viral infections of the gastrointestinal or respiratory tracts and MOGAD[1]. ADEM often occurs in the post-infectious or post-vaccination setting, and an inciting antigenic stimulus has been demonstrated in 67% of cases.[9]
In the setting of recent vaccination, it is believed that the immune adjuvants used to induce an augmented immune response are important in the development of post-vaccination CNS demyelination syndromes, including ADEM.[10] There appears, however, to be a higher risk of CNS demyelination following the infections that the vaccinations immunize against, rather than after the vaccination administration itself.[10] The risk associated with vaccination is relatively low, around 0.1%. It remains unknown whether there is a causal relationship between vaccination and development of ADEM.[2][10]
Risk Factors
The most significant risk factor for developing ADEM is an antecedent viral or bacterial infection, a vaccination.
Identified viral infections associated with ADEM include cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), human herpesvirus 6 (HHV-6), H1N1, hepatitis A, hepatitis C, HIV, influenza, dengue, enterovirus, measles, rubella, coxsackie B, varicella zoster, West Nile virus, SARS COVID infection.[11][12]
Bacterial infectious causes of ADEM include Mycoplasma pneumoniae, Campylobacter jejuni, Chlamydia pneumoniae, Borrelia burgdorferi, Legionella pneumoniae Leptospira spp., and beta-hemolytic Group-A Streptococcus.[11] Among parasites, Falciparum malaria, Vivax malaria, and Toxoplasmosis spp., have been associated with ADEM. Additionally and specially prior to vaccination era, cases of ADEM have been reported after rabies, measles mumps and rubella (MMR), polio (oral), influenza, H1N1, Japanese encephalitis, diphtheria pertussis tetanus (DPT), tetanus toxoid, smallpox, yellow fever, meningococcal, human papilloma virus, and hepatitis B and SARS COVID vaccines. [8][12]
ADEM has also been reported in the post-transplant setting, as well as due to poisoning from the Abrus precatorius plant.[12][13]
More recently ADEM has been associated to MOG-Ab positivity[1]
Pathophysiology
While pathogenesis of ADEM has not been fully delineated, the prevailing theory suggests that antigenic exposure in genetically susceptible individuals could cause the disease through mechanisms involving molecular mimicry and/or T-cell mediated inflammation.[12][14] Molecular mimicry occurs when close homology of an exogenous antigen to host tissue (e.g., myelin sheath) induces an autoimmune reaction to native tissue. Antigenic exposures from infections or vaccines cause the body to form antibodies against epitopes that resemble molecules found in the CNS. Proposed endogenous targets include myelin basic protein (MBP), myelin-associated oligodendrocyte basic protein (MOBP), oligodendrocyte specific protein (OSP), myelin oligodendrocyte glycoprotein (MOG)[1], myelin-associated glycoprotein (MAG), and proteolipid protein (PLP).[15][16] The resultant autoimmune reaction stimulated by autoantibodies has been hypothesized to lead to ADEM.[14]
A second hypothesis involves CNS injury secondary to generalized inflammation from a viral infection. In the priming phase, an offending infection disrupts the blood-brain barrier, allowing host myelin epitopes to enter the peripheral vascular circulation. These myelin epitopes are presented via antigen-presenting cells to T lymphocytes in secondary lymphoid tissues, creating myelin-reactive T lymphocytes that are now poised to attack host myelin upon encountering this endogenous host tissue.[17] In the setting of ongoing inflammation, these myelin-reactive T cells encounter their derivative antigen (host myelin epitopes) on the major histocompatibility class II complexes of antigen-presenting cells in the perivascular spaces. This activates the myelin-reactive T lymphocytes, allowing them to cross into the brain parenchyma. This leads to the effector phase, when activated T cells promote the production of cytokines and chemokines via antigen-presenting cells, recruiting polymorphonuclear phagocytes and monocytes.[18] Proteases and free oxygen radicals released by these leukocytes break down the blood-brain barrier, allowing stronger inflammatory processes to cause demyelination of the CNS. The transmigration of lymphocytes is thought to be further aided by increased expression of adhesion molecules such as Intercellular Adhesion Molecule 1 (ICAM-1) and E-selectin, and higher levels of these molecules have been reported in children with ADEM.[19] Inflammatory, demyelinating processes observed in the animal models that are proposed to drive the pathogenesis of ADEM include the production of tumor necrosis factor-alpha, complement activation, antibody-dependent cell-mediated cytotoxicity (ADCC), myelin phagocytosis, oxygen and nitrogen free radicals, CD8+ cytotoxic T-cell-mediated axonal injury, protease secretion, and oligodendrocyte apoptosis.[17] Evidence supporting an inflammatory component to the pathogenesis includes elevated CSF cytokines and chemokines in patients with ADEM.[2]
Recent research proposes that some patients possess a genetic predisposition to ADEM, which potentially explains why certain individuals may be more likely to develop ADEM following infection or vaccination. Case series in Russia, Korea, and Brazil have shown that children with certain human leukocyte antigen (HLA-DR) subtypes have a higher frequency of ADEM than children with other subtypes.[12]
A key histopathologic finding in ADEM is perivenular sleeves of demyelination, caused by infiltration of inflammatory cells such as macrophages, B and T lymphocytes, plasma cells, and granulocytes. As demyelination continues, these perivenular lesions can grow larger and merge, forming the massive, globular lesions characteristic of ADEM.[20] This is in contrast to the confluent demyelination seen in MS, where sheets of macrophages intermixed with reactive astrocytes infiltrate completely demyelinated areas in a layer-like pattern. Another key difference on biopsy is that the lesions in ADEM will all be in the same stage of demyelination, while in MS both active and inactive lesions exist simultaneously.
Primary prevention
No primary prevention currently exists for ADEM, as the disease entity usually follows routine viral infection or vaccination. No specific guidelines exist for secondary prevention of relapses.[21]
Diagnosis
History
ADEM may resemble other demyelinating diseases on initial presentation, such as MS and NMO. Patients should be asked about the onset, duration, exacerbating factors, and previous neurologic episodes. Additionally, the provider should take note of the patient’s age, as ADEM most commonly presents in young children aged 5 – 8 compared to a median age of 29 in MS. Recent infection or vaccination may raise clinical suspicion for ADEM, although this history finding is not exclusive to the diagnosis. Since the association of MOG-Ab and ADEM is been now identified, a cell based assay exam for MOG-Ab detection is frequently encouraged. Clinicians should maintain a wide differential diagnosis when suspecting a demyelinating process after taking the patient’s history.
Physical Examination
In patients with suspected ADEM, a full neurologic exam should be performed and documented to determine the location, severity, and nature of any neurologic deficit. Additionally, ophthalmic evaluation is indicated if the patient has complaints suggestive of optic neuritis or any other visual pathology. External examination, extraocular movements, pupil reactivity , pupil size and, specially relative Afferent Pupillary Defect (rAPD), pupil size, visual acuity, stereopsis, color vision, and confrontation visual field testing should be performed. The patient should be examined under the slit lamp to rule out any corneal surface or anterior chamber issues that could be causing visual impairment. The fundus should be visualized to look for optic disc edema.
Signs and symptoms
In both children and adults, patients with ADEM present with multifocal neurologic deficits in the setting of encephalopathy (coma, stupor, lethargy) and/or altered behavior (e.g., irritability, confusion). The presence of encephalopathy is a key clinical feature that helps distinguish ADEM from MS. A prodromal syndrome of malaise, fever, headache, nausea, and vomiting may occur, which could represent a viral or bacterial antecedent infection as discussed above. This prodrome typically lasts 3-4 days before onset of neurologic symptoms. [2] The neurologic deficits peak around 2-5 days after onset.[22] Anywhere from 15-25% of children with ADEM require hospital admission in an intensive care unit.
The most commonly reported neurologic symptoms include[9]
- Weakness of lower extremity/upper extremity (17 – 77%)
- Ataxia (10 – 52%)
- Cranial nerve palsies (11 – 48%)
- Optic neuritis (7-23%)
- Seizures (4 – 48%)
- Fever (27 – 63%)
- Headache and/or vomiting (15 – 37%)
- Meningeal signs (13 – 43%)
Patients with ADEM may present with unilateral or bilateral optic neuritis and may complain of subacute vision loss, pain with eye movement, and dyschromatopsia. Optic disc edema may also be present. Bilateral optic neuritis presents more frequently in ADEM than in MS.[9][23] Patients with ADEM or multiphasic DEM (MDEM) can also develop subsequent attacks of optic neuritis. When recurrent optic neuritis follows a diagnosis of ADEM or MDEM, the disease entity is called ADEM-ON.[22] The visual acuity may be poor, with one study showing a median visual acuity of 20/600 in patients with ADEM and optic neuritis.[23] Additionally, 2 case reports have shown peripapillary hemorrhage in patients presenting with optic neuritis.[24][25] Cases of uveitis preceding ADEM have also been reported in a 69-year-old and a 17-year-old girl.[26][27]
Patients with cortical involvement may experience more profound states of encephalopathy, as the cerebral cortex is responsible for higher levels of sensory processing. Lesions affecting the occipital lobe and visual cortex may result in homonymous visual field defects and other cortical visual disturbances, including cortical blindness if severe and bilateral. Additionally, these patients may present with aphasia, alexia, agraphia, or acalculia depending on the location of the lesion. Cortical involvement may lead to a focal loss of motor or sensory function that can be localized to lesions along the motor or sensory homunculus. If the motor cortex is affected, patients can have upper motor neuron signs (hyperreflexia, spasticity, Babinski sign, pronator drift) in the muscle groups supplied by the demyelinated cortical neurons. Symptoms of higher order sensory loss in ADEM, such as agraphesthesia and astereognosia can occur, as well as loss of proprioception, light touch, and pain and temperature.
Patients with brainstem involvement can experience difficulties with functions of the cranial nerves III-XII, as well as dysfunction in the white matter tracts that course through the brainstem. This includes diplopia from cranial nerve palsies or inflammation of the gaze control centers causing impaired extraocular movements. Patients can also experience dysphagia, dysarthria, nystagmus, nausea/vomiting, vertigo, ataxia, hearing impairment, loss of taste and smell, focal motor impairments, impaired consciousness, and respiratory failure.[22] Patients with brainstem involvement usually have a fulminant disease course with a worse prognosis compared with those without lesions in the brainstem.[28][29][30]
Atypical symptoms include persistent headache or meningeal signs, stroke-like events, recurrent seizures, dystonia or Parkinsonism, neuropsychiatric symptoms, progressive onset, and recurrent encephalopathic events.[22]. There is one case report of "pathologic yawning" that is hypothesized to be secondary to brainstem or hypothalamic lesions. Although common in NMOSD, this symptoms is rare in ADEM but resolves with IV steroids.[31]
Diagnostic criteria
According to the International Pediatric Multiple Sclerosis Study Group (IPMSSG), 4 diagnostic criteria are required to make a diagnosis of ADEM in children[32]:
- Multifocal, clinical CNS event with presumed inflammatory demyelinating cause
- Encephalopathy that cannot be explained by fever, systemic illness, or post-ictal fever
- No new clinical and MRI findings 3 months or more after the onset
- Brain MRI is abnormal with changes consistent with demyelination during the acute, 3-month phase
Additionally, a 2019 review article by Cole J et al further divided ADEM into the following subtypes or categories:
- Monophasic ADEM: single ADEM episode with no further demyelinating events more than 3 months after onset.
- Multiphasic ADEM: Two episodes of ADEM separated by at least 3 months in time. More than two episodes suggests an alternate disease process.
- ADEM-ON: Any episode of ADEM plus one or more episodes of optic neuritis. To date, all cases reported have been MOG+.
- Acute hemorrhagic encephalomyelitis (AHEM): Fulminant presentation associated with multifocal hemorrhages and necrosis.
It is important to note that these categorizations are often applied retrospectively, mainly when assessing for relapsing clinical features or changes in brain imaging at follow up encounters. Also, none of these diagnoses are final; should the patient develop additional attacks without encephalopathy or imaging changes remote from the initial attack, they would likely meet criteria for MS. [2]
Finally, while many of these subtypes are defined primarily by time course and features of relapse, AHEM is distinguished by its severity at presentation. MRI typically demonstrates numerous hemorrhages and death can occur in less than 1 day due to cerebral edema or herniation. [2]
Laboratory and diagnostic testing
In patients with clinical symptoms suggestive of ADEM, physicians should initially rule out treatable CNS infections and other causes of CNS demyelination. The studies should include:
- Complete blood count
- Erythrocyte sedimentation rate
- C-reactive protein
- Anti-nuclear antibody(ANA)
- Anti-aquaporin 4 antibody (strongly associated with neuromyelitis optica)
- Myelin oligodendrocyte glycoprotein(MOG) titers
- Herpes simplex virus/ Enterovirus/ Epstein-Barr virus
- Mycoplasma
- COVID19 (from recent reports)
- NM
Of note, based on the current diagnostic guidelines, all patients who meet criteria for ADEM and are also MOG-Ab positive would also meet criteria for MOG-Ab-associated disorder. MOG-Ab titers are present in 33-66% of pediatric cases and are strongly associated with a non-MS relapsing disease course. [2]Almost all patients with ADEM have negative anti-aquaporin 4 antibody (AQP4-Ab) titers, and NMOSD patients that were neg for AQP4, ~40% tested positive for MOG antibodies. [33] The presence of atypical symptoms (see Signs and Symptoms above) should guide diagnostic workup to support or refute less likely etiologies.[22]
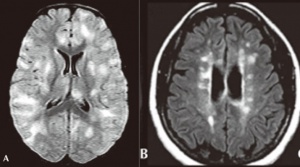
Neuroimaging is helpful in diagnosing ADEM. At presentation, MRI findings may be similar between MS and ADEM. In patients with ADEM, T2-weighted and fluid attenuated inversion recovery (FLAIR) brain MRI typically shows large, bilateral, hyperintense, lesions (>1-2 cm) that are diffuse and poorly demarcated.[22] ADEM lesions can involve both the white and gray matter, affecting the subcortical and central white matter, cortical gray-white matter junction, and deep gray matter of the basal ganglia, thalami, cerebellum, brainstem, with very rare T1 hypointense “black hole” white matter lesions that are more typically seen in MS.[14][22] Gadolinium enhancement of ADEM lesions is found in only 30% of cases.[35]
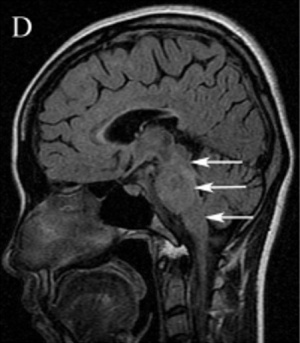
Patients with optic neuritis can have unilateral or bilateral T2 hyperintense lesions of the optic nerve with gadolinium enhancement. The enhancement is usually homogenous, and the nerve will appear thickened and swollen. However, optic nerve enhancement may also demonstrate a heterogeneous pattern, with enhancement of the peripheral fibers of the optic nerve.[37]
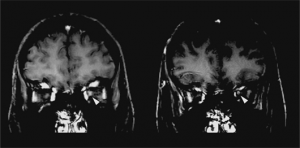
Key brain MRI features found in ADEM that are absent in MS include periventricular sparing without Dawson fingers (ovoid lesions perpendicular to lateral ventricles), both deep gray matter and cortical involvement, bilateral and diffuse lesions that are large, globular, and poorly demarcated.[22] In 1/3 of cases, spinal cord demyelination is seen over multiple segments. Diffusion-weighted imaging (DWI) can help elucidate the timing of the demyelinating event.[39] In the first week following ADEM onset, DWI will show decreased diffusivity. This is thought to be a result of inflammation causing myelin sheath edema and accumulation of inflammatory cells, impeding the migration of water molecules. After the first week, the extracellular space widens, causing increased water diffusivity on DWI.[39]
Lumbar punctures are often obtained in the emergency setting when patients present with encephalopathy. Cerebrospinal fluid (CSF) studies can help differentiate cases of ADEM from MS as CSF in ADEM is often normal and lacks an identifying set of characteristics. The following may be seen in the CSF:
- Leukocyte count is normal in 42-72% of patients in pediatric cohorts with ADEM.[35][3]
- CSF protein is increased in 23 – 62% of patients with ADEM.[35][3]
- Mild pleocytosis, if present, has lymphocytic and monocytic predominance[40][41]
- Rare elevated CSF IgG index[22]
Rare presence of oligoclonal bands found in the CSF compared to MS (0 – 29% in ADEM compared to 82 - 94% of patients with MS).[11][27][42] Of note the presence of oligoclonal bands in ADEM is associated with a higher risk of later developing epilepsy. [2]The levels of Alpha 2-Macroglobulin (α2M), both in the CSF and serum, are also being researched as a biomarker to aid in the diagnosis and evaluation of ADEM. α2M levels in the CSF, as well as the ratio of the α2M in the CSF to that of the serum, were significantly increased in the acute phase of ADEM. They also decreased after steroid pulse treatment. However the study used only a small number of samples and further accumulation of data is warranted. [42]
Abnormal visual evoked responses may be seen, and patients with ADEM are more likely to have abnormal electroencephalography than patients with MS (80% vs. 29%, respectively).[43]Interictal EEG findings are typically abnormal but non-specific; diffuse slowing is the most common finding, although focal spikes and focal slowing have also been reported. [2]
Differential diagnosis
ADEM is a diagnosis of exclusion with several differential diagnoses including vascular, demyelinating, infectious, autoimmune/inflammatory, toxic, metabolic and nutritional disorders. MRI findings in addition to the clinical picture help support a diagnosis of ADEM versus other diagnoses. CSF analysis, oligoclonal bands, serum autoantibodies, and MRI are key diagnostic tools used to narrow the differential diagnosis.[2] This differential diagnosis includes[22][31]
- Vascular disorders (primary CNS vasculitis, systemic lupus erythematosus [SLE] with CNS involvement, prothrombotic state, antiphospholipid antibody syndrome, sickle cell anemia, Moyamoya disease)
- CNS infection (viral, bacterial, or parasitic meningoencephalitis, HIV-associated encephalopathy, subacute sclerosing panencephalitis)
- Intracranial mass lesion (lymphoma, astrocytoma, histiocytosis, gliomatosis cerebri, brain abscesses)
- Autoimmune/inflammatory (MS, NMO spectrum disorders, neurosarcoidosis, Marburg disease, acute demyelinating cerebellitis, acute demyelinating brainstem encephalitis, Neuro-Behçet disease, posterior reversible encephalopathy syndrome)
- Toxic, nutritional, and metabolic disorders (Leigh syndrome, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes [MELAS], B12 deficiency, folate deficiency, central pontine myelinolysis, inherited leukodystrophies)
- Trauma
- Anti-N-methyl-D-aspartate receptor acute disseminated encephalomyelitis-like [44]
Management
General Treatment
ADEM is usually a self-limiting condition that improves spontaneously. Considering that the initial presentation may mimic CNS infection, it is fairly common for children with ADEM to initially receive antimicrobial treatment. However once the diagnosis is clearly established, immunosuppressive therapy is the mainstay of treatment. As of this writing, no double-masked placebo-controlled clinical trial exists that establishes the treatment of choice. [2]
Medical therapy
After a diagnosis of ADEM has been made, intravenous (IV) corticosteroids are generally administered.[14][9] The most common initial treatment regimen is a 3-5 day course of high dose IV glucocorticoids (one option being 10 to 30 mg/kg/day of methylprednisolone, up to a maximum dose of 1000 mg per day, another being 1 mg/kg/day of dexamethasone, which does not have a clear maximum daily dose). It should be noted that few studies exist comparing these two regimens, but one small study which compared methylprednisolone (n = 21) to dexamethasone (n = 25) found a statistically significant improvement in outcome in those treated with methylprednisolone. After the initial IV course, a prolonged oral steroid taper is administered, with a common regimen being prednisone at 1 mg/kg/day, up to maximum 60 mg/day, and then tapered over 4 to 6 weeks.[2]
Should the patient have a contraindication to steroids, another acute treatment option is IVIG. The total dose is 1 to 2 g/kg, given either as a single dose (usually of 1 g/kg) or divided over 3-5 days (usually 400mg/kg/day).
In patients refractory to these treatments, plasma exchange should be performed immediately after treatment failure occurs.[14][45][46] Some case reports have shown the use of hypothermia or decompressive craniotomy for treatment of fulminant ADEM with a life-saving outcome.[28][47]
Acute hemorrhagic leukoencephalitis is an acute, severe form of ADEM that can be rapidly fatal from hours to days without treatment and may require a combination of all of the above treatments.[45] Cyclophosphamide has been used to treat steroid-unresponsive ADEM in a few adult patients, but optimal dosing has not been established.[21] It should be noted that no randomized controlled trials exist to prove superiority of any treatment over placebo.
Finally, due to the possibility of relapse, as well as the diagnosis of a modified subtype of ADEM or even a completely different diagnosis altogether (e.g. MS), clinicians should establish a plan for clinical and radiographic follow up after the acute event. However, the optimal frequency for follow-up imaging has not been clearly established. Among children with recurrent MOG-Ab-positive syndromes such as MDEM or ADEM-ON, immunomodulatory drugs such as azathioprine, mycophenolate mofetil, and rituximab have been shown to reduce, but unfortunately not eliminate, relapses. Regularly scheduled IVIG infusions have also been shown to reduce relapse frequency and have been associated with clinical improvement over time. [2]
Prognosis
Patients with ADEM typically have a good prognosis. Most children with ADEM recover fully with a return to neurologic baseline within weeks (57 – 89%), with some studies even citing 31 days or less.[22][45] It should be noted that one study showed longer follow up time was related to better scores in attention domains of neuropsychological testing. However it is important to consider differences in outcome measures; studies that assessed subtler, especially cognitive findings, were more likely to report longer and lower rates of recovery time. [2] Approximately 20-30% show residual neurologic deficits, most commonly focal motor deficits (e.g., clumsiness, ataxia, hemiparesis), visual problems, and epilepsy.[35] Of patients diagnosed with ADEM, 1-3% pass away due to complications of a severe disease course. Additionally, permanent cognitive impairments have been observed in the domains of executive function, verbal processing, attention, and IQ scores.[48] This impact is more frequent in children under 5 years of age. Adults with ADEM have increased rates of hospitalization, intensive care unit admission, and mortality compared to pediatric patients.[49] The rate of progression from ADEM to MS is relatively low, with incidence reports between 0 and 17% in various studies with follow up periods lasting years.
Further studies are suggested by some authors in order to understand the possible neurological long term manifestations associated with COVID-19.
Conclusion
In conclusion, ADEM is an acute, autoimmune, demyelinating disease of the CNS with a broad differential diagnosis that affects predominantly pediatric patients without a strong biological sex predisposition. Features that help point towards a diagnosis of ADEM include:
- Encephalopathy in addition to focal neurologic deficits
- Large, bilateral globular, poorly demarcated cortical white matter and deep gray matter T2-weighted and FLAIR lesions on MRI that spare the periventricular region
- Recent history of immunization or infection.
Diagnostic workup includes laboratory evaluation for inflammatory and infectious etiologies as well as MRI to look for areas of demyelination. Lumbar puncture typically shows a normal cell count and no oligoclonal bands, but a mild pleocytosis may exist. The current mainstay of treatment is a regimen of IV corticosteroids, although evidence is lacking to support this treatment over other modalities. A plan for follow up to assess clinical status, imaging changes, and possible progression to different diagnoses is an important component of management, although definitive guidelines do not yet exist. The prognosis of ADEM is usually good, with the majority of children returning to baseline mental status and neurologic function within a few weeks. About 20-30% of children experience permanent neurologic deficits, with some evidence showing impairment in the higher domains of executive function and verbal processing. In 1-3% of cases, patients pass away from a severe disease course. Multicenter studies are needed to further characterize the pathogenesis, biomarkers, and treatment options to provide the best long-term therapy for minimizing deleterious patient outcomes.
References
- ↑ 1.0 1.1 1.2 1.3 Ambrosius W, Michalak S, Kozubski W, Kalinowska A. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management. Int J Mol Sci. 2020;22(1):100. Published 2020 Dec 24. doi:10.3390/ijms22010100
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 Cole J et al., Acute Disseminated Encephalomyelitis in Children: An Updated Review Based on Current Diagnostic Criteria, Pediatric Neurology, https://doi.org/10.1016/j.pediatrneurol.2019.06.017
- ↑ 3.0 3.1 3.2 3.3 Torisu H, Kira R, Ishizaki Y, et al. Clinical study of childhood acute disseminated encephalomyelitis, multiple sclerosis, and acute transverse myelitis in Fukuoka Prefecture, Japan. Brain Dev. 2010;32(6):454-462.
- ↑ 4.0 4.1 Pohl D, Hennemuth I, Kries R von, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr. 2007;166(5):405-412.
- ↑ Banwell B, Kennedy J, Sadovnick D, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009;72(3):232-239.
- ↑ Absoud M, Lim MJ, Chong WK, et al. Paediatric acquired demyelinating syndromes: incidence, clinical and magnetic resonance imaging features. Mult Scler Houndmills Basingstoke Engl. 2013;19(1):76-86.
- ↑ Pellegrino P, Radice S, Clementi E. Geoepidemiology of Acute Disseminated Encephalomyelitis: Epidemiology. 2014;25(6):928-929.
- ↑ 8.0 8.1 Anilkumar AC, Foris LA, Tadi P. Acute Disseminated Encephalomyelitis. [Updated 2022 Jul 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430934/
- ↑ 9.0 9.1 9.2 9.3 Koelman DLH, Mateen FJ. Acute disseminated encephalomyelitis: current controversies in diagnosis and outcome. J Neurol. 2015;262(9):2013-2024.
- ↑ 10.0 10.1 10.2 Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13(3):215-224.
- ↑ 11.0 11.1 11.2 Tenembaum S, Chitnis T, Ness J, Hahn JS, International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):S23-26.
- ↑ 12.0 12.1 12.2 12.3 12.4 Garg RK, Malhotra HS, Kumar N. Chapter 10 - Pathophysiology of Acute Disseminated Encephalomyelitis. In: Minagar A, ed. Multiple Sclerosis. San Diego: Academic Press; 2016:201-248.
- ↑ Ninan, E. C., & James, E. (2019). Acute disseminated encephalomyelitis due to abrus precatorius poisoning – A case report. Saudi Pharmaceutical Journal, 27(4), 521–524. https://doi.org/10.1016/j.jsps.2018.11.016
- ↑ 14.0 14.1 14.2 14.3 14.4 Hussein O, Minagar A. Acute Disseminated Encephalomyelitis: Clinical Features, Pathophysiology, and Clinical Management. In: Inflammatory Disorders of the Nervous System. Current Clinical Neurology. Humana Press, Cham; 2017:161-173.
- ↑ Schirmer L, Srivastava R, Hemmer B. To look for a needle in a haystack: the search for autoantibodies in multiple sclerosis. Mult Scler J. 2014;20(3):271-279.
- ↑ Stonehouse M, Gupte G, Wassmer E, Whitehouse WP. Acute disseminated encephalomyelitis: recognition in the hands of general paediatricians. Arch Dis Child. 2003;88(2):122-124.
- ↑ 17.0 17.1 Sospedra M, Martin R. Immunology of Multiple Sclerosis*. Annu Rev Immunol Palo Alto. 2005;23:683-747.
- ↑ Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11(3):328-334.
- ↑ Martino D, Branson JA, Church AJ, et al. Soluble Adhesion Molecules in Acute Disseminated Encephalomyelitis. Pediatr Neurol. 2005;33(4):255-258.
- ↑ Young NP, Weinshenker BG, Parisi JE, et al. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010;133(2):333-348.
- ↑ 21.0 21.1 Pohl D, Tenembaum S. Treatment of Acute Disseminated Encephalomyelitis. Curr Treat Options Neurol. 2012;14(3):264-275.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 Pohl D, Alper G, Haren KV, et al. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 Suppl 2):S38-S45.
- ↑ 23.0 23.1 Dale RC, de Sousa C, Chong WK, Cox TCS, Harding B, Neville BGR. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(12):2407-2422.
- ↑ Mendonca N, Shah AJ, Saldanha M, Gonsalves SRJ. Acute disseminated encephalomyelitis presenting as optic neuritis in a case of idiopathic thrombocytopaenic purpura. BMJ Case Rep. 2014;2014.
- ↑ Chan WH, Lloyd IC, Ashworth JL, et al. Acute disseminated encephalomyelitis associated with optic neuritis and marked peri-papillary hemorrhages. Eye. 2011;25(12):1658-1659.
- ↑ Tan PY, Furness J, Sohal AS, Ramesh V, Haider S. A case of intermediate uveitis as a precursor to acute disseminated encephalomyelitis (ADEM) in a teenager. Eye. 2014;28(5):625-627.
- ↑ 27.0 27.1 R Tsutsumi, Fukushima A, Hayashi N, Nishino K, Koura Y, Komatsu T. A case of uveitis followed by acute disseminated encephalomyelitis. Jpn J Clin Ophthalmol. 2005;59(4):471-474.
- ↑ 28.0 28.1 Miyamoto K, Kozu S, Arakawa A, et al. Therapeutic Hypothermia With the Use of Intracranial Pressure Monitoring for Acute Disseminated Encephalomyelitis With Brainstem Lesion: A Case Report. J Child Neurol. 2014;29(9):NP69-NP73.
- ↑ Tenembaum SN. Disseminated encephalomyelitis in children. Clin Neurol Neurosurg. 2008;110(9):928-938.
- ↑ Donmez FY, Aslan H, Coskun M. Evaluation of possible prognostic factors of fulminant acute disseminated encephalomyelitis (ADEM) on magnetic resonance imaging with fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging. Acta Radiol. 2009;50(3):334-339.
- ↑ 31.0 31.1 Birca V, Saint-Martin C, Myers KA. Teaching Video Neuroimages: Pathologic yawning A sign of brainstem involvement in acute disseminated encephalomyelitis? Neurology May 2020, 10.1212/WNL.0000000000009595; DOI: 10.1212/WNL.0000000000009595
- ↑ Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler J. 2013;19(10):1261-1267.
- ↑ Narayan R, Simpson A, Fritsche K, et al. MOG antibody disease: A review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2018;25:66‐72. doi:10.1016/j.msard.2018.07.025
- ↑ Lee YJ. Acute disseminated encephalomyelitis in children: differential diagnosis from multiple sclerosis on the basis of clinical course. Korean J Pediatr. 2011;54(6):234-240. doi:10.3345/kjp.2011.54.6.234
- ↑ 35.0 35.1 35.2 35.3 Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: A long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224-1231.
- ↑ Lu Z, Zhang B, Qiu W, et al. Comparative Brain Stem Lesions on MRI of Acute Disseminated Encephalomyelitis, Neuromyelitis Optica, and Multiple Sclerosis. PLoS ONE. 2011;6(8).
- ↑ Gala F. Magnetic resonance imaging of optic nerve. Indian J Radiol Imaging. 2015;25(4):421-438.
- ↑ Hirayama T, Ikeda K, Hidaka T, Nagata R, Yoshii Y, Kawabe K, Iwasaki Y. Unilateral Measles-Associated Retrobulbar Optic Neuritis without Encephalitis: A Case Report and Literature Review. Case Rep Neurol. 2010;2:128-132.
- ↑ 39.0 39.1 Kamr WH, El-Tantawy AM, Moustafa M, Abd-Elsalam OA. Acute disseminated encephalomyelitis: MR Diffusion weighted imaging: Potential diagnostic value and outcome predilection. Egypt J Radiol Nucl Med. 2017;48(1):215-223.
- ↑ Hung P-C, Wang H-S, Chou M-L, Lin K-L. Acute disseminated encephalomyelitis in children: a single institution experience of 28 patients. Neuropediatrics. 2012;43(2):64.
- ↑ Leake JAD, Albani S, Kao AS, et al. Acute Disseminated Encephalomyelitis in Childhood: Epidemiologic, Clinical and Laboratory Features: Pediatr Infect Dis J. 2004;23(8):756-764.
- ↑ 42.0 42.1 Suzuki Y et al., Ratio of Alpha 2-Macroglobulin Levels in Cerebrospinal Fluid and Serum: An Expression of Neuroinflammation in Acute Disseminated Encephalomyelitis, Pediatric Neurology, https://doi.org/10.1016/j.pediatrneurol.2019.04.020
- ↑ Brass SD, Caramanos Z, Santos C, Dilenge M-E, Lapierre Y, Rosenblatt B. Multiple sclerosis vs acute disseminated encephalomyelitis in childhood. Pediatr Neurol. 2003;29(3):227-231.
- ↑ Lekoubou A, Viaccoz A, Didelot A, et al. Anti-N-methyl-D-aspartate receptor encephalitis with acute disseminated encephalomyelitis-like MRI features. Eur J Neurol. 2012;19(2):e16-e17. doi:10.1111/j.1468-1331.2011.03617.x
- ↑ 45.0 45.1 45.2 Tenembaum SN. Acute disseminated encephalomyelitis. In: Handbook of Clinical Neurology. Vol 112. Elsevier; 2013:1253-1262.
- ↑ Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878-886.
- ↑ Granget E, Milh M, Pech-Gourg G, et al. Life-saving decompressive craniectomy for acute disseminated encephalomyelitis in a child: a case report. Childs Nerv Syst. 2012;28(7):1121-1124.
- ↑ Jacobs RK, Anderson VA, Neale JL, Shield LK, Kornberg AJ. Neuropsychological outcome after acute disseminated encephalomyelitis: Impact of age at illness onset. Pediatr Neurol. 2004;31(3):191-197.
- ↑ Ketelslegers I, Visser I, Neuteboom R, Boon M, Catsman-Berrevoets C, Hintzen R. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler J. 2011;17(4):441-448.

