Onchocerciasis (African River Blindness)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Onchocerciasis, otherwise known as “African River Blindness,” is a filarial infection caused by the nematode Onchocerca volvulus that can lead to visual morbidity via multiple mechanisms including chorioretinitis, uveitis, and sclerosing keratitis. It manifests systemically but is best known for its cutaneous and ocular pathologies.
Disease Entity
Onchocerciasis ICD-9 125.3. This article focuses on the ophthalmic consequences of this systemic disease.
Disease
Onchocerciasis (“African River Blindness”) is a filarial infection caused by the nematode Onchocerca volvulus—one of the nine worldwide filarial nematodes in which humans are the definitive host. Of the three subtypes associated with filarial infection (lymphatic, subcutaneous, and visceral), onchocerciasis falls into the subcutaneous category, as one of its main presenting features are subcutaneous nodules.[1] These nodules are followed in turn by ocular manifestations that, if left untreated, can lead to blindness.
Prevalence and Incidence
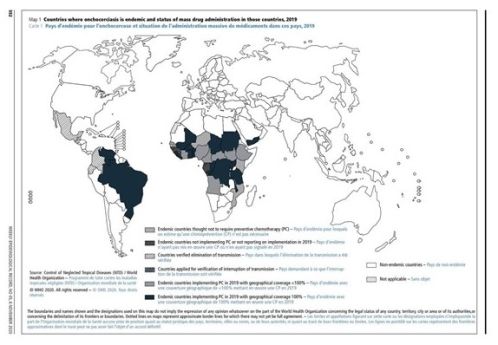
Onchocerciasis is the second leading cause of blindness due to infection in the world.[3] The Global Burden of Disease Study estimated that in 2017 there were at least 20.9 million people are infected worldwide, of which 14.6 million had skin disease and 1.15 million had vision loss .[1][4] [5] [6] [7] The infection is found in tropical climates with the main burden of disease concentrated in 31 countires in sub Saharan Africa. It is also found in limited ares of South America and in Yemen in the Middle East.[1][4][5][8] It should be noted, however, that public health efforts by public and private partnerships involving organizations such as the the Expanded Special Project for the Elimination of Neglected Tropical Diseases in Africa (ESPEN) and the Carter Center have resulted in significant progress, especially in Latin America. Four countries have been verified by WHO as free of onchocerciasis after successfully implementing elimination activities for decades: Colombia (2013), Ecuador (2014), Mexico (2015), and Guatemala(2016).[9] However, it remains that more than 90 million people are estimated to be at risk in Africa, with Nigeria and Democratic Republic of the Congo (previously known as Zaire) being the most affected countries.[6][10] [11]In these highly endemic areas, infection rates can be as high as 80-100% by 20 years of age, with clinical manifestations usually peaking at 40-50 years of age.[6][12] [13]These infections often result in major socioeconomic liabilities, as working-aged adults are often the ones debilitated, leaving the young to care for the adults as well as to provide for the family. Hyperendemic regions are frequently depopulated due to a 3-4 time increase in all-cause mortality compared to non-infected populations, and average decrease in life expectancy by 7-12 years.[1][6][14] [15] [16] [17] As of 2024, at least 249.5 million people in 28 countries require interventions to eliminate onchocerciasis. This total does not include people living in areas in which transmission status is unknown. By 2024, MDA had been stopped in 221 implementation units (IU) that had been previously affected by active transmission, with 18.8 million people requiring preventive chemotherapy (PC) for onchocerciasis, which have either been completed or are under post-treatment surveillance (PTS). Most are in Africa. In 2023, a total of 172.2 million people were treated for onchocerciasis, representing 69.0% global coverage. [18]
Etiology
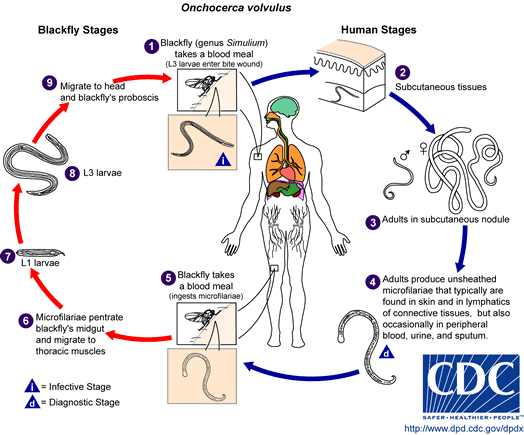
Onchocerca volvulus breed exclusively in the Simulium yahense black fly, found only in certain endemic areas near fast-flowing rivers and streams. The life cycle starts by a black fly biting an infected human host and ingesting microfilariae from the skin or blood. These microfilariae migrate from the gut to the thoracic flight muscles of the fly, where they develop from the initial larval phase into the infective microfilariae after one week (Stages J1 --> J2). They then migrate to the salivary glands of the fly and are ready to be transmitted again (Stage J3s).[1][3][19]
The third stage larvae are then introduced into the skin of the new human host via a fly bite, where over the next 6-12 months, they develop into mature adult parasites. The larger female nematode migrates to the subcutaneous or deeper fascial tissues and becomes encapsulated by a fibrous shell. The males migrate into these capsules in order to fertilize the females. It is these capsules that are the characteristic subcutaneous nodules of onchocerciasis. Fertilized adult females in these nodules produce millions of microfilariae, which are responsible for the systemic and ocular findings of onchocerciasis.[19] While the most common mode of transmission is direct via insect bites, the disease can also be spread transplacentally from an infected mother to her fetus.[4]
Risk Factors
Exposure to the Simulium yahense black fly in endemic areas.
Pathophysiology
The multiple sequelae result from direct invasion of the nematodes, which leads to induction of inflammation in the infected host. Eventually, these inflammatory changes can lead to blindness through corneal sclerosing keratitis, secondary glaucoma, cataracts, chorioretinitis, and optic atrophy.
While still alive, O. volvulus cause very little in terms of pathology. The adult worms are protected by the fibrous skin nodules in which they reside, and the microfilariae (through some unknown mechanism) are non-immunogenic. It is when they die that they cause an immune response. Antigens that are released by dead or dying organisms cause a T-helper cell (TH2) response, which leads to the release of interleukins, resulting in the influx of neutrophils and eosinophils, and the production of antibodies by plasma cells. These inflammatory responses lead to corneal opacification.[1][4] Specifically, it is thought that the sclerosing keratitis is an effect of modification of ICAM-1 expression and production of IL-4 and IL-14.[20]
More recent research suggests that it is largely the Wolbachia bacteria (which are endosymbionts of O. volvulus that are released upon the death of the microfilariae), which cause the immunogenic response.[21] [22] [23] [24]As evidence to this point, in Africa there are two main strains of O. volvulus: one located near the savannas, and the other near the rainforests. Of the two, the savanna strain is more likely to cause ocular disease, even with only moderate parasite burdens, where the other, the rainforest strain, does not cause blindness despite high parasite burdens. This propensity for ocular disease has been correlated to higher quantities of Wolbachia DNA in the savanna strain, shown by PCR.[25] [26] [27]
The associated iridocyclitis is thought to result from this inflammatory response, which in turn can lead to cataract formation. Similarly, the glaucoma associated with onchocerciasis is often thought to be due to synechiae formation as a secondary form of angle closure glaucoma.[4] However, onchocerciasis has been found to be an independent risk factor in glaucoma even in those without synechiae formation. Light microscopy studies have shown affected individuals to have normal trabecular anatomy, but abnormal post-trabecular anatomy affecting the more downstream outflow system. This is thought to be a factor in the increased prevalence of glaucoma in endemic areas despite the lack of synechiae formation in affected individuals.[28]
Interestingly, the ocular inflammation associated with onchocerciasis is thought to be due in part to antigen mimicry, especially in the posterior pole. Recent studies suggest a role for cross-reactivity between the O. volvulus antigen Ov39 and the retinal antigen hr44 as a possible cause for the continued progression of chorioretinitis in some individuals despite decreased microfilariae burden and ongoing treatment.[29] [30] [31] [32] [33] [34] [35]
Primary Prevention
Global preventative efforts are currently concentrated in population-based prophylactic treatment through programs such as the African Programme for Onchocerciasis Control (APOC) which launched in 1995 and closed in 2015 and the Expanded Special Project for the Elimination of Neglected Tropical Diseases in Africa (ESPEN) started in 2016.[5][11] Community-wide ivermectin administration (Mectizan®, donated by Merck) and vector (black fly) control have been the mainstay of these programs, which have been markedly successful. In APOC’s final year, more than 119 million people were treated with ivermectin,. With support from ESPEN, ivermectin treatments continued to scale up, reaching 152.9 million people in 2019, but due to COVID-19 disruptions, the number of people treated declined by 26.9% in 2020.[5][8][13][36]. However, in some highly endemic areas, more than three decades of annual treatment are still needed in order to effectively control this preventable disease.[8] Individual prevention can include protective measures to avoid contact with the black flies in endemic areas, including avoiding areas in which the disease is systemic, using insect repellent and wearing appropriate clothing.[1][19][37]
Diagnosis
Diagnosis is made clinically with confirmation by superficial skin snip biopsies. Newer adjuvant tests including PCR amplification and serology have largely supplanted the historical Mazzotti test (see below, “Medical Treatment”).[1][4]
History
The majority of patients would have lived in endemic areas. The classic history includes preceding dermatologic lesions associated with severe pruritus, which leads to ocular signs and symptoms several years later. Other systemic symptoms result from invasion of the CNS, viscera, and urinary systems.
Physical Examination
Slit lamp examination can be used to visualize the ocular findings associated with Onchocerciasis. Occasionally, the microfilaria in the anterior chamber or cornea can be visualized, aiding in diagnosis. The nematodes can be seen using retro-illumination as S or C-shaped, fine motile elements. Patients are asked to sit with their head between their knees for at least 2 minutes in order to mobilize the microfilariae that are suspended in the anterior chamber, increasing the sensitivity of this test. [4][37][38]
These ocular findings are most often accompanied by cutaneous findings, warranting a full dermatologic exam. These can include freely mobile subcutaneous nodules measuring 0.5 – 3.0 cm in diameter most often palpable over bony prominences (especially over the hips and lower limbs or head). These nodules present concurrently with the development of diffuse papular dermatitis, orange-peel textured skin, lichenified dermatitis, atrophy, fine wrinkles, lymphadenitis ("hanging groin"), and eventually depigmentation (“leopard skin”).[1]
Signs
Typically, dermatologic manifestations are the initial presenting symptoms. Ophthalmic signs often present several years afterwards. The severity of these ocular symptoms depends on the duration of infection and the microfilarial load, as well as the strain of microfilaria that is infecting the host.[22][23][27]
O. volvulus involves all ocular tissues. Initially, it can involve the eyelid and conjunctiva, leading to eyelid nodules and edema, chronic conjunctivitis, chemosis, and phlyctenule-like kerato-conjunctival lesions. Then, by direct invasion, microfilariae infect the cornea and sclera.
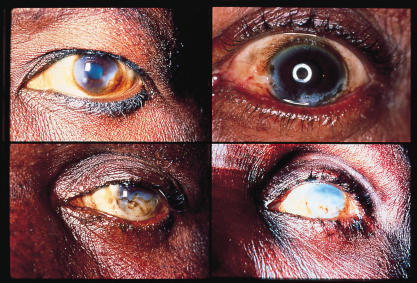
Corneal manifestations include fine interpalpebral sub-epithelial punctate lesions. These “snowflake opacities” can lead to a chronic sclerosing keratitis and discrete nummular scars with stromal edema, corneal infiltration, and neovascularization. It is the sclerosing keratitis that contributes to this nematode's permanent blinding effect.
The microfilariae invade the iris and the ciliary body, leading to iridocyclitis that can be severe. This can result in corectopia, iris atrophy and extensive synechiae that can result in secondary glaucoma, as well as early cataract formation.[1][4][37][38]
Posterior chamber involvement can include chorioretinal lesions. Peripapillary chorioretinitis can result in optic nerve dysfunction secondary to optic nerve edema and optic neuritis, eventually leading to optic atrophy.[28][33]
Symptoms
Typically, patient present with cutaneous symptoms including itching and dermatologic changes years before the onset of ocular symptoms. These symptoms can be very severe, leading to excoriation, bleeding, and even suicide.[1][19]In terms of ocular disease, patients will classically complain of an insidious onset of decreased vision, eye redness, eye pain and photophobia related to iridocyclitis, and perceived corneal changes related to the sclerosing keratitis. These changes often manifest in the patients’ 4th to 5th decade. [4][19][37]
Clinical Diagnosis
Slit lamp examination can be diagnostic or suggestive of the disease. Visualizing nematodes in the anterior chamber can demonstrate the presence of a parasitic infection. However, typically nematodes are not visualized, and the classic corneal, anterior chamber, and chorioretinal lesions in the setting of an appropriate history and antecedent skin findings can lead to the correct diagnosis.
Laboratory tests
Confirmation of suspected onchocerciasis involves a bloodless skin snip where specimens are taken from areas of apparently involved skin, as well as from the scapula, over each iliac crest and each calf. These specimens are then incubated for up to 24 hours in normal saline and a stain is applied in order to identify the species of the motile elements seen in the specimens (O. volvulus have no sheath or nuclei in their tails, differentiating them from other nematodes). This test is very specific but not sensitive for those with lower worm burdens, early in their infection. It is more valuable 18+ months post-infection for the worms to be abundant, large and mature enough to be detected.[38]
Serology can also be used to detect exposure to O. volvulus with high sensitivity and specificity. Newer ELISA and Western Blot techniques have been used to quantitatively detect antibodies to O. volvulus antigens in the skin, tears, and urine. Other assays detect levels of antibody subclass IgG4 for diagnosis.[37][38]PCR, ultrasound of the nodules, and sclerocorneal biopsy have also been suggested as other tests to help confirm the diagnosis.[19]
Proposed tests that are currently being investigated include the diethylcarbamazine (DEC) skin patch test, the antibody card test, and the antigen detection test.[5][39] [40] [41] However, these tests are not currently available for clinical use and are not commonly available in endemic areas.
More antiquated methods include using the Mazzotti test, in which DEC (diethylcarbamazine) is given to individuals who are thought to be infected, leading to systemic reactions including rashes, fevers, generalized body pains, uveitis, and possible anaphylaxis. The presence of these findings were highly suggestive of onchocerciasis (as well as other filarial infections), and the killed microfilariae could sometimes be found in the blood or urine. However, due to the severity of the reactions, it is no longer used clinically.[1][19][38]
Differential diagnosis
The differential diagnosis of the dermatologic findings, in isolation from the ophthalmic findings, is extensive. The diffuse papular dermatitis may be due to food allergies, syphilis, leprosy, vitamin A deficiency, and yaws.[1][19][38] The differential diagnosis for subcutaneous nodules is also extensive, and can include: multiple types of tumors (mesenchymal, metastatic, etc.), skin appendage lesions, inflammatory or infectious lesions, or other tumor-like lesions or cysts.[42]
In the context of the characteristic ocular findings, the differential includes infection with other microfilaria (including Mansonella perstans, Loa loa, Onchocerca gutturosa, or Dracunculus medinensis), inflammatory lesions involving iridocyclitis including systemic inflammatory etiologies such as sarcoidosis, and other corneal degenerative/sclerotic diseases.[4][27][43] [44]
Management
Medical Therapy
Traditionally, DEC (diethylcarbamazine) was given to kill the microfilariae, leading to the systemic side effects of the Mazzotti test (see Diagnosis, above). However, this drug was phased out of use by the development of newer antihelminthics without the same spectrum of systemic side effects.
The standard treatment of onchocerciasis now utilizes the broad-spectrum antiparasitic ivermectin, given as a single dose and repeated every 6-12 months over a course of around 10 years. Ivermectin decreases the microfilarial load by paralyzing the microfilariae over a six-month period. It has no effect on the adult nematodes, however. Monotherapy with ivermectin (150µg/kg) has been shown to mitigate the development of optic atrophy when treatment is started early in the course of the disease.[8][13][38]Moreover, ivermectin has been shown to reduce the visual field loss associated with onchocerciasis years after treatment, as well as the severity of keratitis. However, more advanced chorioretinal and keratitis lesions, and secondary glaucoma are not treated by ivermectin. Acute complications that result from the infection can be treated symptomatically—e.g. any iridocyclitis can be treated with topical corticosteroids or cycloplegic medications.
Antibiotic trials using 6-week-long courses of doxycycline against the endosymbiont, Wolbachia have shown decreased microfilarial loads in adults.[24] Moreover, it was found that treating the worms with doxycycline prior to the death of the host nematode reduced the numbers of Wolbachia and resulted in a decrease in post-treatment corneal thickness and stromal haze in an animal model. It can also suppress reproduction of the adult nematodes and production of microfilariae for up to 18 months. However, trials are lacking on shorter durations of treatment.
Recent literature has suggested the appearance of strains of filarial nematodes similar to O. volvulus and strains of O. volvulus that are resistant to ivermectin.[45] [46] [47] [48] [49]Therefore, other treatments have been tried with varying levels of success include rifampin, rifampicin[47], and azithromycin.[50] Further study is needed to investigate alternative therapies for the treatment of onchocerciasis.
Medical follow up
Patients suspected to have onchocerciasis should be referred to an infectious disease specialist familiar with the management of this mostly sub-Saharan tropical disease. Those undergoing treatment for any ophthalmic complications should be followed frequently, at least every 3-6 months initially, to ensure adequate control of any ongoing inflammation or other complications that may arise. However, no standardized protocol exists and follow-up should be scheduled at the discretion of the treating physician.
Surgery
Surgical interventions can be directed at the underlying etiology of vision loss. Cataract extraction can be performed on visually significant cataracts induced by the disease process. Trabeculectomy or other filtering procedures can be performed as needed for secondary glaucoma. Corneal pathology can potentially be treated with penetrating keratoplasty (PKP), and chorioretinal lesions may be treated by vitrectomies or laser photocoagulation as indicated.
Surgical follow up
There is no established protocol for surgical follow-up of onchocerciasis patients. Follow-up should be determined by the treating physician and be based on the clinical picture and type of procedure performed.
Sequelae
The ocular sequelae are manifold. As mentioned before, the infection can lead to corneal opacification (sclerosing keratitis), secondary angle closure and open angle glaucoma, cataracts, chorioretinitis, and optic atrophy. Combined, these manifestations lead to the blindness that gives the disease its name.
Prognosis
Prognosis depends on the stage at which the infection is treated. If treated early during the course of the disease, the corneal opacities may be minimal and may recede with appropriate treatment. However, as the disease progresses, the visual outcomes can progress towards blindness and may be irreversible.
In a global sense, studies have found a direct relationship between O. volvulus microfilarial load and early host mortality.[14][15][16][17]Other studies have shown significant socioeconomic and public health effects of the disease. Overall, this is a devastating disease with a multitude of ramifications that bears a poor prognosis for the villages and the residents of these villages that are afflicted by this terrible disease.
However, it should be noted that major strides have been made through the combined efforts of public and private sector partnerships, involving such organizations as the Carter Center, Helen Keller International, Lions Clubs International, Onchocerciasis Control Programme (OCP), and African Programme for Onchocerciasis Control (APOC), and the Expanded Special Project for the Elimination of Neglected Tropical Diseases in Africa (ESPEN), in conjunction with local health ministries. As a result of this work, it was recently announced that parts of Uganda and Sudan have broken the cycle of transmission of onchocerciasis.[51] [52] The progress made in these previously endemic areas serves as an inspiration for countries in which the burden of disease is great and challenges the preconception that this is a disease that will forever afflict these disadvantaged persons.
Additional Resources
- http://www.who.int/blindness/partnerships/onchocerciasis_OCP/en/
- http://www.who.int/apoc/onchocerciasis/ocp/en/
- http://www.cartercenter.org/health/river_blindness/index.html
- Pham HH, Harrison DA. Onchocerciasis (African River Blindness). American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/onchocerciasis-african-river-blindness. Accessed March 20, 2019.
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Diemert, D.J. Tissue Nematode Infections. Goldman’s Cecil Medicine 193-200 (2011).doi:10.1016/B978-1-4377-1604-7.00574-1
- ↑ https://www.who.int/en/news-room/fact-sheets/detail/onchocerciasis
- ↑ Jump up to: 3.0 3.1 Dent, A.E. & Kazura, J.W. Other Tissue Nematodes. Nelson Textbook of Pediatrics 1225-1227 (2011).doi:10.1016/B978-1-4377-0755-7.00289-X
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Mcleod, S.D. Parasitic Keratitis. Yanoff & Duker: Ophthalmology 274-8 (2008).doi:10.1016/B978-0-323-04332-8.00038-X
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 Richards, F.O., Boatin, B., Sauerbrey, M. & Sékétéli, A. Control of onchocerciasis today: status and challenges. Trends Parasit 17, 558-63 (2001).
- ↑ Jump up to: 6.0 6.1 6.2 6.3 WHO Expert Committee on Onchocerciasis Control Onchocerciasis and its Control. (Geneva, 1995).
- ↑ Basañez, M.-G. et al. River blindness: a success story under threat? PLoS medicine 3, e371 (2006).
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Cupp, E.W., Sauerbrey, M. & Richards, F. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan(®)) monotherapy. Acta tropica 120 Suppl, S100-8 (2011).
- ↑ The Carter Center River Blindness (Onchocerciasis) Program [Internet]. [cited 2012 Aug 9] Available from: http://www.cartercenter.org/health/river_blindness/index.html
- ↑ WHO | Water-related Diseases. at http://www.who.int/water_sanitation_health/diseases/oncho/en/
- ↑ Jump up to: 11.0 11.1 WHO | Status of onchocerciasis in APOC countries. at http://www.who.int/apoc/onchocerciasis/status/en/index.html
- ↑ Greene, B.M. Modern medicine versus an ancient scourge: progress toward control of onchocerciasis. J Infect Dis 166, 15-21 (1992)
- ↑ Jump up to: 13.0 13.1 13.2 Duerr, H.P., Raddatz, G. & Eichner, M. Control of onchocerciasis in Africa: threshold shifts, breakpoints and rules for elimination. Int J Parasit 41, 581-9 (2011).
- ↑ Jump up to: 14.0 14.1 Little, M.P., Breitling, L.P., Basáñez, M.-G., Alley, E.S. & Boatin, B. a Association between microfilarial load and excess mortality in onchocerciasis: an epidemiological study. Lancet 363, 1514-21 (2004).
- ↑ Jump up to: 15.0 15.1 Kirkwood, B., Smith, P., Marshall, T. & Prost, A. Relationships between mortality, visual acuity and microfilarial load in the area of the Onchocerciasis Control Programme. Trans R Soc Trop Med Hyg 77, 862-8 (1983).
- ↑ Jump up to: 16.0 16.1 Prost, A. & Vaugelade, J. Excess mortality among blind persons in the West African savannah zone. (Article in French). Bull World Health Organ 59, 773-6 (1981).
- ↑ Jump up to: 17.0 17.1 Prost, A. The burden of blindness in adult males in the savanna villages of West Africa exposed to onchocerciasis. Trans Ophthalmol Soc United Kingdom 80, 525-7 (1986).
- ↑ WHO REFERENCE NUMBER: WER No 41, 2024, 99, 577–590
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 Fox, L.M. Blood and Tissue Nematodes (Filarial Worms). Principles and Practice of Pediatric Infectious Diseases: Revised Reprint 1312-1317 (2009).doi:10.1016/B978-0-7020-3468-8.50284-4
- ↑ Berger, R.B., Blackwell, N.M., Lass, J.H., Diaconu, E. & Pearlman, E. IL-4 and IL-13 regulation of ICAM-1 expression and eosinophil recruitment in Onchocerca volvulus keratitis. Inv Ophthalmol Vis Sci 43, 2992-7 (2002).
- ↑ Cross, H.F., Haarbrink, M., Egerton, G., Yazdanbakhsh, M. & Taylor, M.J. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet 358, 1873-5 (2001).
- ↑ Jump up to: 22.0 22.1 Keiser, P.B. et al. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J Infect Dis 185, 805-11 (2002).
- ↑ Jump up to: 23.0 23.1 Higazi, T.B. et al. Wolbachia endosymbiont levels in severe and mild strains of Onchocerca volvulus. Mol Biochem Parasit 141, 109-12 (2005).
- ↑ Jump up to: 24.0 24.1 Hoerauf, A. et al. Depletion of wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment For personal use . Only reproduce with permission from The Lancet Publishing Group . Lancet 357, 415-1416 (2001).
- ↑ Zimmerman, P.A. et al. Onchocerca volvulus DNA probe classification correlates with epidemiologic patterns of blindness. J Infect Dis 165, 964-8 (1992).
- ↑ Erttmann, K.D., Meredith, S.E., Greene, B.M. & Unnasch, T.R. Isolation and characterization of form specific DNA sequences of O. volvulus. Acta Leidensia 59, 253-60 (1990).
- ↑ Jump up to: 27.0 27.1 27.2 Meredith, S.E., Lando, G., Gbakima, A.A., Zimmerman, P.A. & Unnasch, T.R. Onchocerca volvulus: application of the polymerase chain reaction to identification and strain differentiation of the parasite. Exp Parasit 73, 335-44 (1991).
- ↑ Jump up to: 28.0 28.1 Egbert, P.R., Jacobson, D.W., Fiadoyor, S., Dadzie, P. & Ellingson, K.D. Onchocerciasis: a potential risk factor for glaucoma. Br J Ophthalmol 89, 796-8 (2005).
- ↑ Semba, R.D. et al. Longitudinal study of lesions of the posterior segment in onchocerciasis. Ophthalmology 97, 1334-41 (1990).
- ↑ Chippaux, J.P. et al. Effect of repeated ivermectin treatments on ocular onchocerciasis: evaluation after six to eight doses. Ophthal Epid 6, 229-46 (1999).
- ↑ Neumann, E. & Gunders, A.E. Pathogenesis of the posterior segment lesion of ocular onchocerciasis. Am J Ophthalmol 75, 82-9 (1973).
- ↑ Anderson, J., Fuglsang, H., Marshall, T.F. & Al., E. Studies on onchocerciasis in the United Cameroon Republic. IV. A four-year follow-up of six rain-forest and six savanna villages. The incidence of ocular lesions. Trans R Soc Trop Med Hyg 2, 513-5 (1978).
- ↑ Jump up to: 33.0 33.1 Murphy, R.P., Taylor, H. & Greene, B.M. Chorioretinal damage in onchocerciasis. Am J Ophthalmol 98, 519-21 (1984).
- ↑ Braun, G., McKechnie, N.M., Connor, V. & Al., E. Immunological crossreactivity between a cloned antigen of Onchocerca volvulus and a component of the retinal pigment epithelium. J Exp Med 174, 169-77 (1991).
- ↑ McKechnie, N.M., Gürr, W., Yamada, H., Copland, D. & Braun, G. Antigenic mimicry: Onchocerca volvulus antigen-specific T cells and ocular inflammation. Inv Ophthalmol Vis Sci 43, 411-8 (2002).
- ↑ https://www.who.int/en/news-room/fact-sheets/detail/onchocerciasis
- ↑ Jump up to: 37.0 37.1 37.2 37.3 37.4 Freedman, D.O. Onchocerciasis. Tropical Infectious Diseases: Principles, Pathogens and Practice 1176-88 (2005).
- ↑ Jump up to: 38.0 38.1 38.2 38.3 38.4 38.5 38.6 Murdoch, M.E. Onchocerciasis. Up To Date (2012).at http://www.uptodate.com/contents/onchocerciasis
- ↑ Weil, G.J., Lammie, P.J. & Weiss, N. The ICT Filariasis Test: A rapid-format antigen test for diagnosis of bancroftian filariasis. Parasit Today 13, 401-4 (1997).
- ↑ Weil, G.J. et al. A rapid-format antibody card test for diagnosis of onchocerciasis. J Infect Dis 182, 1796-9 (2000).
- ↑ Stingl, P., Ross, M., Gibson, D.W., Ribas, J. & Connor, D.H. A diagnostic “patch test” for onchocerciasis using topical diethylcarbamazine. Trans R Soc Trop Med Hyg 78, 254-8 (1984).
- ↑ Beaman, F.D. et al. Superficial soft-tissue masses: analysis, diagnosis, and differential considerations. Radiographics : a review publication of the Radiological Society of North America, Inc 27, 509-23 (2007).
- ↑ Albiez, E.J., Büttner, D.W. & Duke, B.O. Diagnosis and extirpation of nodules in human onchocerciasis. Trop Med Parasit (German) 39 Suppl 4, 331-46 (1988).
- ↑ Udall, D.N. Recent updates on onchocerciasis: diagnosis and treatment. Clin Infect Dis 44, 53-60 (2007).
- ↑ Coles, G.C., Rhodes, A.C. & Wolstenholme, A.J. Rapid selection for ivermectin resistance in Haemonchus contortus. Vet Parasitol 129, 345-7 (2005).
- ↑ Vickers, M., Venning, M., McKenna, P.B. & Mariadass, B. Resistance to macrocyclic lactone anthelmintics by Haemonchus contortus and Ostertagia circumcincta in sheep in New Zealand. New Zealand Vet J 49, 101-5 (2001).
- ↑ Jump up to: 47.0 47.1 Specht, S. et al. Efficacy of 2- and 4-week rifampicin treatment on the Wolbachia of Onchocerca volvulus. Parasi Res 103, 1303-9 (2008).
- ↑ Osei-Atweneboana, M.Y. et al. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl Trop Dis 5, e998 (2011).
- ↑ Osei-Atweneboana, M.Y., Eng, J.K.L., Boakye, D. a, Gyapong, J.O. & Prichard, R.K. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet 369, 2021-9 (2007).
- ↑ Hoerauf, A. et al. Effects of 6-week azithromycin treatment on the Wolbachia endobacteria of Onchocerca volvulus. Parasit Res 103, 279-86 (2008).
- ↑ Uganda's Success Against River Blindness: An Inspiration for Africa and an International Challenge [Internet]. [cited 2012 Aug 10] Available from: www.cartercenter.org/news/pr/uganda-rb-022312.html
- ↑ Abu Hamad First to Stop River Blindness Transmission in Sudan [Internet]. [cited 2012 Aug 10] Available from: http://www.cartercenter.org/news/pr/abu-hamad-051712.html