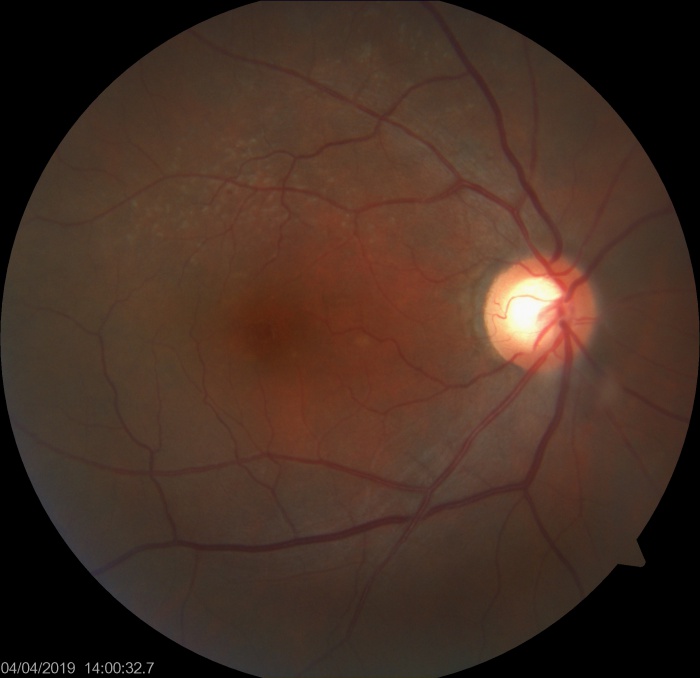
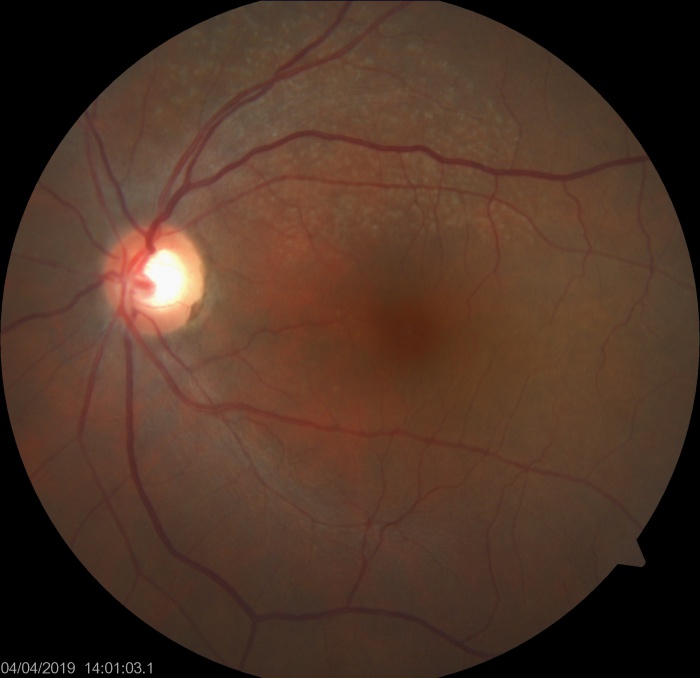
Reticular Drusen
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Reticular drusen, also known as reticular pseudo-drusen (RPD), or subretinal drusenoid deposits, or reticular macular disease were first described by Mimoun et al as “les pseudo-drusen visibles en lumiere bleue” in 1990, which refers to drusen-like material that is more prominent in blue light. Contrary to the drusen which lie below the retinal pigment epithelium (RPE), RPD are located superficial to the RPE. Arnold and colleagues named these lesions as reticular pseudodrusen.[1]
Reticular drusen are yellowish subretinal lesions arranged in a network (i.e. reticular) and are more commonly found at the superotemporal quadrant of the macula.
Epidemiology
- RPD were present in 24% of eyes and 29% of patients with Age-related macular degeneration (ARMD)[2]
- Similar to drusen, it is more prevalent among the advanced age group.
- They are commonly bilateral and symmetrical
- Moreover, it is more common to be seen in female than in male.[3]
Clinical features
RPD appear as dot like whitish-yellow subretinal structures in a cluster which are usually connected in a reticular pattern. Ill defined network of interwoven ribbons may be noted. These are more prominent on blue light channel of fundus photograph. Area superior to the fovea (which has highest rod photoreceptor density) is most commonly involved.[3] The fovea however, is typically spared. The reticular pattern may not be needed for diagnosis, however most studies have required at least 5 RPD lesions and multiple imaging modalities for confirmation of the diagnosis. The RPD can disappear at a specific area of retina with time.
A clinical classification (by Suzuki, Sato, and Spaide[4]) of RPD has divided it into 3 types-
- Dot pseudodrusen- 'hyporeflective spots, often with a target configuration, in IR-SLO images' (infrared scanning laser ophthalmoscope, the auhors used Heidelbeg Spectralis machine). This subtype was detected more with IR-SLO than color fundus images.
- Ribbon pseudodrusen- 'interconnected bands of yellowish-white material forming a reticular pattern, called ribbon pseudodrusen, which were located in the perifovea. This subtype was faintly hyporeflective in IR-SLO imaging'. This type was noted more frequently with the color fundus photo rather than IRSLO.
- Peripheral pseudodrusen-'An uncommon third type of pseudodrusen, yellow-white globules primarily located peripheral to the perifoveal region, appeared hyperreflective in IR-SLO and were called peripheral pseudodrusen.'
RPD are most commonly seen in association with ARMD. RPD may be isolated also.
Other associations of RPD include
- Pseudoxanthoma elasticum
- Sorsby macular dystrophy
- Acquired vitelliform lesion
Other possible associations inclde
- Vitamin A deficiency
- Retinitis punctata albescens
- fundus albipunctatus
- Complement-mediated immunoglobulin A nephropathy
Testing and evaluation
- Reticular drusen was regarded as one of the abnormal autofluorescence patterns in early age-related macular degeneration (AMD) by Bindewald and associates.
- These lesions are usually seen with biomicroscopic fundal examination but remains undetected in fundus fluorescein angiography
- Besides direct visualization of lesions with indirect microscopy, reticular drusen can also be delineated with imaging modalities such as near-infrared photography with scanning laser ophthalmoscope, fundus autofluorescence and indocyanine green angiography.
- Although reticular drusen can be seen with biomicroscopy, it is not readily identifiable in the neurosensory retina removed histological specimen of choroid as reported by Arnold and associates. Arnold et al were unable to isolate these reticular drusen, however, they found that the presence of reticular drusen was associated with significant choroidal thinning. As a result, Arnold concluded that these reticular pseudo-drusen were manifestations of choroidal ischaemia and fibrosis. The nature of reticular drusen was revealed by the studies conducted by Rudolf and Zweifel. Rudolf et al identified subretinal drusenoid deposits in their histological specimen and they found that these subretinal lesions shared characteristics of classical soft drusen located between retinal pigment epithelium (RPE) and Bruch's membrane. However, no clinical correlations were identified at that time. With the introduction of the repeatable, non-invasive optical coherence tomography, high resolution cross-sectional imagining of the retinal and choroidal layers became readily available, and retinal and choroidal conditions can therefore be evaluated and quantified. Zweifel and colleagues offered spectral domain optical coherence tomography (sd-OCT) scans to patients with clinical reticular drusen. They re-examined one of the histological specimen previously reported by Rudolf et al and try to correlate the sd-OCT and histological findings and concluded that reticular drusen are actually subretinal drusenoid deposits with components similar to classical AMD drusen, and are located between the RPE and the photoceptor inner segment/outer segment junction. The presence of reticular drusen has later been proven to be a risk factor for late AMD, both in the disease eye and in the fellow eye.
Investigations
- Reticular pseudodrusen are multiple yellowish-white lesions arranged in a reticular network pattern. Color fundus photo and even blue channel images may not be very sensitive to pick up RPD.
- On OCT scans, these lesions are shown as granular hyperreflective deposits situate between the RPE layer and the ellipsoid zone. OCT has been used to classify RPD into 3 types[5] by Zweifel and colleagues -
- Type 1- hyperreflective material between RPE and Inner segment-outer segment junction or ellipsoid zone (EZ). There is no elevation or breach of EZ.
- Type 2- the hyperreflective material accumulates and forms a mound over the RPE with distortion of overlying EZ
- Type 3- the hyperrefelctive material has a conical configuration which punctures the EZ and reaches outer retina.
- On red-free photography, subretinal drusenoid deposits are light lesions arranged in a network pattern.
- On fundus autofluorescence imaging, reticular drusen are shown up as numerous spots of reduced autofluorescence, with brighter lines in-between.
- On OCT scans, these lesions are shown as granular hyperreflective deposits situate between the RPE layer and the ellipsoid zone. OCT has been used to classify RPD into 3 types[5] by Zweifel and colleagues -
- Similarly, reticular drusen are hypofluorescent lesions on mid- to late-phase of indocyanine green angiography.
- On near-infrared photography, reticular drusen are hyporeflectant lesions on a hyperreflectant background. On red-free photography, subretinal drusenoid deposits are light lesions arranged in a network pattern.
Differential diagnosis
- Cuticular drusen or Basal laminar drusen- These are subretinal structures, and fundus fluroescein angiogram typically shows starry sky appearance, whereas RPDs are very difficult to appreciate in fluorescein angiogram.
- Laser marks on the retina- Usually macular laser spots have gaps in between. Also, the indication of macular laser may be visible on clinical examination and the patient will give history of retinal laser.
Associations and prognosis
RPDs are associated with
- geographic atrophy
- choroidal neovascularization (CNV) especially type 3 CNV may have particular association with RPD
- outer retinal atrophy
References
- ↑ Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina 1995;15(3):183–191.
- ↑ Domalpally A, Agrón E, Pak JW, et al. Prevalence, Risk, and Genetic Association of Reticular Pseudodrusen in Age-related Macular Degeneration: Age-Related Eye Disease Study 2 Report 21. Ophthalmology. 2019;126(12):1659–1666. doi:10.1016/j.ophtha.2019.07.022
- ↑ Jump up to: 3.0 3.1 Wightman AJ, Guymer RH. Reticular pseudodrusen: current understanding. Clin Exp Optom. 2019;102(5):455–462. doi:10.1111/cxo.12842
- ↑ Suzuki M, Sato T, Spaide RF. Pseudodrusen subtypes as delineated by multimodal imaging of the fundus. Am J Ophthalmol. 2014;157(5):1005–1012. doi:10.1016/j.ajo.2014.01.025
- ↑ Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117(2):303–12.e1. doi:10.1016/j.ophtha.2009.07.014
Further reading
- Mimoun G, Soubrane G, Coscas G. Macular Drusen [Article in French]. J Fr Ophtalmol. 1990;13(10):511-30.
- Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G.Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007 Mar;91(3):354-9.
- Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008 Feb;145(2):317-326.
- Joachim N, Mitchell P, Rochtchina E, Tan AG, Wang JJ. Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology. 2014 Apr;121(4):917-25.
- Bindewald A, Bird AC, Dandekar SS, et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Invest Ophthalmol Vis Sci. 2005 Sep;46(9):3309-14.
- Arnold JJ, Quaranta M, Soubrane G, Sarks SH, Coscas G. Indocyanine green angiography of drusen. Am J Ophthalmol. 1997 Sep;124(3):344-56.
- Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15(3):183-91.
- Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008 Nov;87(5):402-8.
- Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010 Feb;117(2):303-12.e1.
- Maguire MG, Fine SL. Reticular pseudodrusen. Retina. 1996;16(2):167-8.
- Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration.Invest Ophthalmol Vis Sci. 2006 Dec;47(12):5495-504.
- Finger RP, Wu Z, Luu CD, et al. Reticular Pseudodrusen: A Risk Factor for Geographic Atrophy in Fellow Eyes of Individuals with Unilateral Choroidal Neovascularization. Ophthalmology. 2014 Feb 8. [Epub ahead of print]
- Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011 Jul;95(7):979-85.
- Schmitz-Valckenberg S, Alten F, Steinberg JS, et al; Geographic Atrophy Progression (GAP) Study Group. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011 Aug 24;52(9):5009-15.