Microcephaly and Chorioretinopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
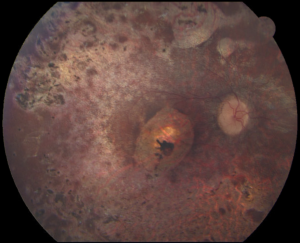
Microcephaly and chorioretinopathy is a group of rare genetic conditions affecting the growth of the head and eyes in utero. Autosomal recessive microcephaly and chorioretinopathy (MCCRP) results from biallelic pathogenic variants in three genes (TUBGCP4, TUBGCP6, and PLK4), while pathogenic variants in KIF11 lead to autosomal dominant microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR). There have been few reported cases of each entity but congenital microcephaly and chorioretinopathy are constant features. Microcephaly is the term for a smaller-than-normal head, typically measured from a head circumference more than two standard deviations below the mean for age and sex. Microcephaly ranges from mild to severe with variable impact on cognitive function, from normal intelligence to moderate developmental delay. Chorioretinopathy is typically described as punched out lesions with possible rod-cone dysfunction, but can sometimes be accompanied by familial exudative vitreoretinopathy (FEVR)-like posterior segment pathology such as retinal folds that can progress to retinal detachment. Other ocular findings may include nystagmus, microphthalmia, high hyperopia, and significant visual impairment.
Genetic Classification and Clinical Features
TUBGCP6 - MCCRP1
Autosomal recessive microcephaly and chorioretinopathy 1 (MCCRP1) is caused by homozygous or compound heterozygous pathogenic variants in the TUBGCP6 gene on chromosome 22q13.
The phenotype of microcephaly and chorioretinopathy was first described in 1966 by Victor McKusick primarily from characterization of a consanguineous Mennonite family, and studies later attributed this family’s condition to TUGCP6 genetic variants.[1][2] Ages at the time of report range from fetal (in utero) to 21 years old. All patients have microcephaly, with many reports of short stature or poor growth. There is a range of developmental delay and intellectual disability from mild to severe in all patients. Other neurologic features can include seizures or abnormal EEG, brisk deep tendon reflexes, hypo or hypertonicity, and shuffling gait reported in the eight related Mennonite patients.[1][2][3][4]
Common dysmorphic features include sloping forehead and high palate. Broad nasal bridge, micro- or retrognathia, cupped ears, and small anterior fontanelle were reported in at least two patients. Musculoskeletal features include digit anomalies and scoliosis or kyphosis.[1][2][3][4]
Chorioretinal or retinal atrophy, retinal degeneration, and/or rod-cone dystrophy are the most common ocular features.[1][2][3][4][5] The eight related patients in the original McKusick study were also all noted to have an abnormally pigmented fundus.[1] Other common ophthalmic findings include nystagmus, microphthalmia and/or microcornea, strabismus, retinal detachment associated with vitreoretinopathy and/or retinal folds, and diminutive vasculature or lack of peripheral vascularization.[2][3][4][5]
Brain MRI findings when reported are notable for pachygyria or simplified gyral pattern, and corpus callosum dysgenesis was also reported in at least 2 patients. There were three brain MRI reports demonstrating just microcephaly or with normal findings.[2][3][4] Cardiac anomalies including small atrial septal defect, ventricular septal defect, and patent foramen ovale were reported in three patients.[2][3][4]
PLK4 - MCCRP2
Autosomal recessive microcephaly and chorioretinopathy 2 (MCCRP2) is caused by heterozygous compound or homozygous pathogenic variants in the polo-like kinase 4 (PLK4 gene) on chromosome 4q28.
There have been 14 reported cases of PLK4-related MCCRP2, half of which represent two different consanguineous families.[6][7][8][9][10] The primary phenotype overlaps with Seckel syndrome, and includes severe microcephaly, global developmental delays, short stature or dwarfism, and microphthalmia.[6][7][8][9][10] Facial dysmorphism consists of sloping forehead, prominent nose, prominent eyes, and micrognathia.[6][7][8][9][10] Despite its namesake, the retinopathy in MCCRP2 has not been fully characterized.[6]
The most common neuroimaging feature, when obtained, is markedly reduced cortical volume with simplified gyral folding, but there have been reports of a small cerebellum, small brainstem, colpocephaly or lissencephaly, and bilateral asymmetric periventricular nodular heterotopia.[6][7][8][9][10]
Other ophthalmic features include pale optic discs, thin retinal vessels, microcornea, cataracts, optic nerve hypoplasia, persistent hyperplastic primary vitreous, and strabismus.[6][7][8][9][10]
TUBGCP4 - MCCRP3
Autosomal recessive microcephaly and chorioretinopathy 3 (MCCRP3) is caused by compound heterozygous pathogenic variants in the TUBGCP4 gene on chromosome 15q15.
There have been nine reported cases of MCCRP3.[3][11][12][13][14] Ages at the time of report range from 4 years old to 19 years old. All have microcephaly and, when reported, mild to moderate decreased intellectual ability. MRI of the brain is often unremarkable but pachygyria and lissencephaly, thin corpus callosum, and delayed myelination were separately reported. Facial dysmorphia has been reported but no one particular feature has been identified as a constant.[3][11][12][13][14]
Chorioretinopathy is described as punched out lesions with pigmentary changes and atrophy, which is reported in all cases. Other common ocular features include nystagmus, microphthalmia, high hyperopia, and retinal folds or bands. ERG response is typically poor with subnormal and delayed photopic and scotopic response or non-recordable responses. Visual acuity ranges from 20/80 to 20/2000 in eyes without retinal detachment, of which two have been reported. Optic nerve pallor or hypoplasia have been noted.[3][11][12][13][14]
Other systemic features include a patient each with a horse-shoe kidney, Tetralogy of Fallot, intrauterine growth restriction, and acanthosis nigricans.[11][12][13]
KIF11 - MCLMR
Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR) is a rare autosomal dominant condition caused by pathogenic variants in the kinesin family member 11 (KIF11) gene on chromosome 10q23.33.
Predominant features include microcephaly, chorioretinopathy, lymphedema, and intellectual disability. Microcephaly is congenital with slow head growth in infancy, and is often associated with mild to moderate learning impairment. KIF11 variants are not always associated with typical facial features, but when present, the characteristic facial phenotype can include upslanting palpebral fissures, broad nose with rounded tip, long philtrum with thin upper lip, prominent ears, and sometimes microretrognathia.[3][15][16][17] MRI features vary and have included normal brain parenchyma, delayed myelination, simplified gyri, increased or prominent extra-axial space, mildly thin corpus callosum, partial agenesis of the corpus callosum, and foreshortened frontal lobe.[18] Epilepsy has been reported rarely.[18][19]
Chorioretinopathy is the most common eye abnormality and is described as lacunae of extramacular chorioretinal atrophy with pigment clumping and vessel attenuation, although diffuse chorioretinal atrophy that includes the posterior pole has been described.[3][15][17] Other ophthalmic features include hyperopia or hyperopic astigmatism, and more rarely myopia or myopic astigmatism; microphthalmia; microcornea; optic disc pallor or dysplasia; cataracts; and retinal folds and detachments. Recent studies have recognized a phenotypic overlap between MCLMR and familial exudative vitreoretinopathy (FEVR) related to KIF11 variants.[3][20][21][22][23][24][25] A wide range of visual acuities have been reported, from 20/25 to 20/20000, depending on the extent of dysplasia.[17]
Lymphedema, when present, is typical congenital, bilateral, and confined to the lower limbs, although adult onset lymphedema has also been reported. There have been a few reports of concomitant cardiac abnormalities including congenital thickened pulmonary valve, atrial septal defect, and patent foramen ovale.[15][16][17]
Etiology/Pathophysiology
Primary microcephaly has been attributed to defects in neurogenesis, with a majority of elucidated microcephaly-related genes encoding centrosomal proteins.[15][26][27][28] Abnormal centrosomal function can result in problems with centriole duplication, spindle assembly, microtubule dynamics, and cell cycle regulation, which all can impact neuronal progenitor proliferation and migration of developing neurons during neurogenesis.[26][27][28]
The four genes known to cause microcephaly and chorioretinopathy (TUBGCP4, TUBGCP6, PLK4, and KIF11) all encode proteins involved in microtubule dynamics and mitotic spindle function. During mitosis, microtubules are nucleated by the centrosome, which comprises a centriole pair surrounded by pericentriolar material, a protein matrix studded with ring-shaped γ-tubulin structures termed the γ-tubulin ring complex (γ-TuRC).[29] The γ-TuRC is composed of γ-tubulin with γ-tubulin complex proteins GCP2, GCP3, GCP4, GCP5, and GCP6.[30] GCP4 and GCP6 incorporate into the ring structure and have been shown to be critical to γ-tubulin targeting at the centrosome, and reduction in activity or function leads to defects in centriole duplication, spindle assembly, and mitotic arrest.[31][32][33]
Molecular analysis suggests reduced GCP4 function leads to destabilization or disassembly of γ-TuRCs.[11][34][35] Cell studies have shown that GCP4-haploinsufficiency results in abnormal spindle assembly in mitosis and increased nuclear anomalies in interphase.[11][34][35] Another report characterizing heterozygous TUBCP4 +/- mice suggests they may demonstrate a retina and microcephaly phenotype due to haploinsufficiency, though this is not a phenotype clinically observed in human heterozygous carriers of TUBGCP4 pathogenic variants.[11][34][35] Notably, total knockout of TUBGCP4 in mice results in early embryonic lethality.[34][35] Consistent with this animal model finding, no humans with biallelic null (total loss of function) TUBGCP4 alleles have been reported, and it is presumed this would not be compatible with embryonic development and viability. Instead, all cases of MCCRP3 have been due to compound heterozygous variants with a common synonymous variant c.1746G>T that induces exon skipping, along with an additional severe null (loss of function) allele.[11][13]
Molecular studies show GCP6 is indispensable to the assembly of γ-TuRC and for microtubule nucleation.[31] GCP6 is also the substrate of the PLK4 kinase, which has been termed the master regulator of centriole duplication.[6][29][31] In patient-derived fibroblasts, reduced PLK4 function leads to impaired centriole duplication and reduced centriole number, with subsequent abnormal mitotic spindle formation, delayed mitotic progression, and significant cell division abnormalities overall.[6][35][36] Plk4-morphant zebrafish exhibit dwarfism and variable reduction in eye size, in line with the MCCRP2 clinical phenotype.[6]
KIF11 encodes a homotetramer kinesin motor, which has been shown to play a critical role in spindle assembly and function.[26][37] Animal and cell studies show that inhibition of KIF11 leads to monopolar microtubule spindle formation and chromosome misalignment, which leads to cell cycle arrest and apoptosis, in neural progenitor cells and embryonic neuroretinal cells.[26] KIF11 inhibition in chick embryos and zebrafish models confirm the MCLMR phenotype of microcephaly, chorioretinal dysgenesis, microphthalmia, developmental disorder, and also highly abnormal vasculature.[26] KIF11 pathogenic variants have been associated with a FEVR-like phenotype (see differential diagnosis), and there is evidence that KIF11 plays a critical role in retinal vasculature development.[38][39]
Differential Diagnosis
- TORCH infection: the combination of microcephaly and chorioretinopathy is concerning for a TORCH infection such as CMV, toxoplasmosis, lymphocytic choriomeningitis, or Zika virus. TORCH infections typically present with other systemic findings such as jaundice, hepatosplenomegaly, seizures, intracranial calcifications, and hearing loss. Congenital Zika syndrome will cause severe microcephaly with a partially collapsed skull as well as somewhat similar punched out chorioretinal atrophic lesions.
- FEVR: Fundus features in microcephaly and chorioretinopathy, particularly in KIF11 associated disease, can present with a phenotype similar to Familial Exudative Vitreoretinopathy (FEVR), a heterogenic group of inherited retinal diseases characterized by incomplete vascularization of the peripheral retina due to abnormal retinal angiogenesis.[3][20][24][25]
- Aicardi syndrome: An X-linked syndrome thought to be lethal in male embryos that presents with the classic triad of infantile spasms, chorioretinal lacunae, and partial or total agenesis of the corpus callosum.
- Gyrate atrophy: Peripheral chorioretinal punched-out lesions can resemble the chorioretinopathy of MCCRP and MCLMR, but visual symptoms usually do not begin until late childhood. Additionally, microcephaly is not an associated feature.
- Seckel syndrome: the MCCRP2 phenotype overlaps with Seckel syndrome, a rare genetic condition with intrauterine growth restriction, low birth weight, dwarfism, microcephaly, and intellectual ability.
Management
Genetic variants leading to microcephaly and chorioretinopathy are typically detected by whole exome sequencing, but with increased awareness of the conditions, targeted testing can be performed in cases of high clinical suspicion. Additionally, the causative genes are also now included on standard genetic panels for inherited retinal diseases. ERG testing can be helpful if other inherited retinal conditions are among the differential diagnosis. Since KIF11 pathogenic variants can lead to abnormal peripheral retinal vascular development similar to patients with FEVR, peripheral retinal imaging and even wide field fluorescein angiography should be considered.[20][24] As in FEVR, subsequent laser treatment of the avascular zones may help avoid tractional retinal detachment and prevent further vision loss. If concern for microcephaly and chorioretinopathy arises in the perinatal period, testing for TORCH infections should be undertaken, as these congenital conditions can have systemic effects. Children should be followed closely for routine management of refractive error, amblyopia, and strabismus to help maximize visual potential. These children benefit from low vision services including Orientation and Mobility training early on, as well as multi-disciplinary care with neurology specialists.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 McKusick VA, Stauffer M, Knox DL, and Clark DB. Chorioretinopathy with Hereditary Microcephaly. Arch Ophthalmol, 1966;75:597-600.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Puffenberger EK, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One. 2012;7(1):e28936.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 Shurygina MF, Simonett JM, Parker MA, et al. Genotype Phenotype Correlation and Variability in Microcephaly Associated With Chorioretinopathy or Familial Exudative Vitreoretinopathy. Invest Ophthalmol Vis Sci. Nov 2 2020;61(13):2.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Thomas-Wilson A, Schacht JP, Chitayat D, Blaser S, Santos FJS, Glaser K, et al. Biallelic variants in TUBGCP6 result in microcephaly and chorioretinopathy 1: Report of four cases and a literature review. Am J Med Genet A. 2023;191(7):1935-1941.
- ↑ 5.0 5.1 Hull S, Arno G, Ostergaard P, Pontikos N, Robson AG, Webster AR, et al. Clinical and Molecular Characterization of Familial Exudative Vitreoretinopathy Associated with Microcephaly. Am J Ophthalmol. 2019;207:87-98.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Martin C-A, Ahmad I, Klingseisen A, Hussain MS, Bicknell LS, Leitch A, et al. Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure, and retinopathy. Nature Genetics, 2014;46:1283-1292.
- ↑ 7.0 7.1 7.2 7.3 7.4 Martin-Rivada A, Pozo-Roman J, Guemes M, Ortiz-Cabrera NV, Perez-Jurado LA, and Argente J. Primary Dwarfism, Microcephaly, and Chorioretinopathy due to a PLK4 Mutation in Two Siblings. Horm Res Paediatr. 2020;93(9-10):567-572.
- ↑ 8.0 8.1 8.2 8.3 8.4 Shaheen R, Al Tala S, Almoisheer A, and Alkuraya FS. Mutation in PLK4, encoding a master regulator of centriole formation, defines a novel locus for primordial dwarfism. J Med Genet. 2014;51(12):814-6.
- ↑ 9.0 9.1 9.2 9.3 9.4 Tsutsumi M, Yokoi S, Miya F, Miyata M, Kato M, Okamoto N, et al. Novel compound heterozygous variants in PLK4 identified in a patient with autosomal recessive microcephaly and chorioretinopathy. Eur J Hum Genet. 2016;24(12):1702-1706.
- ↑ 10.0 10.1 10.2 10.3 10.4 Xu Y-S, Su Z-Y, Sun L, Yang X-M, Sun S-Y, Ji X-F, et al. Overexpression of the PLK4 Gene as a Novel Strategy for the Treatment of Autosomal Recessive Microcephaly by Improving Centrosomal Dysfunction. J Mol Neurosci. 2021;71(12):2618-2627.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 Scheidecker S, Etard C, Haren L, et al. Mutations in TUBGCP4 alter microtubule organization via the γ-tubulin ring complex in autosomal-recessive microcephaly with chorioretinopathy. Am J Hum Genet. Apr 2 2015;96(4):666-74. doi:10.1016/j.ajhg.2015.02.011
- ↑ 12.0 12.1 12.2 12.3 Da Palma MM, Motta FL, Takitani G, Salles MV, Lima LH, Ferraz Sallum JM. TUBGCP4 - associated microcephaly and chorioretinopathy. Ophthalmic Genet. Apr 2020;41(2):189-193. doi:10.1080/13816810.2020.1747084
- ↑ 13.0 13.1 13.2 13.3 13.4 Yahalom C, Woods RL, Akula JD, Tan WH, Fulton A. Microcephaly and chorioretinopathy associated with TUBGCP4: a case report and a review of the literature. Ophthalmic Genet. Apr 10 2023:1-6. doi:10.1080/13816810.2023.2170424
- ↑ 14.0 14.1 14.2 Bayram-Suverza M, Torres-Navarro KA, Hernandez-Vazquez AY, Ramirez-Estudillo JA. Microcephaly and Chorioretinopathy Relevance as a Differential Diagnosis. Diagnostics (Basel). Aug 3 2023;13(15)doi:10.3390/diagnostics13152588
- ↑ 15.0 15.1 15.2 15.3 Ostergaard P, Simpson MA, Mendola A, Vasudevan P, Connell FC, van Impel A, et al. Mutations in KIF11 cause autosomal-dominant microcephaly variability associated with congenital lymphedema and chorioretinopathy. Am J Hum Genet. 2012;90(2):356-62.
- ↑ 16.0 16.1 Alahmadi G, Alshamrani AA, Albakri A. Novel variant of KIF11 associated with MCLMR syndrome. Ophthalmic Genet. 2023;44(2):205-207.
- ↑ 17.0 17.1 17.2 17.3 Balikova I, Robson AG, Holder GE, Ostergaard P, Mansour S, and Moore AT. Ocular manifestations of microcephaly with or without chorioretinopathy, lymphedema, or intellectual disability (MCLID) syndrome associated with mutations in KIF11. Acta Ophthalmol. 2016;94(1):92-8.
- ↑ 18.0 18.1 Mirzaa GM, Enyedi L, Parsons G, Collins S, Medne L, Adams C, et al. Congenital microcephaly and chorioretinopathy due to de novo heterozygous KIF11 mutations: five novel mutations and review of the literature. Am J Med Genet A. 2014;164A(11):2879-86.
- ↑ Jones GE, Ostergaard P, Moore AT, Connell FC, Williams D, Quarrel O, et al. Microcephaly with or without chorioretinopathy, lymphoedema, or mental retardation (MCLMR): review of phenotype associated with KIF11 mutations. Eur J Hum Genet. 2014;22(7):881-7.
- ↑ 20.0 20.1 20.2 Robitaille JM, Gillett RM, LeBlanc MA, Gaston D, Nightingale M, Mackley MP, et al. Phenotypic overlap between familial exudative vitreoretinopathy and microcephaly, lymphedema, and chorioretinal dysplasia caused by KIF11 mutations. JAMA Ophthalmol. 2014;132(12
- ↑ Chang Y, Zhang X, Xu K, Li Nien, Xie Y, Yan W, et al. Phenotype-Based Genetic Analysis Reveals Missing Heritability of KIF11-Related Retinopathy: Clinical and Genetic Findings. Genes (Basel). 2023;14(1):212.
- ↑ Chen C, Sun L, Li S, Huang L, Zhang T, Wang Z, et al. Novel variants in familial exudative vitreoretinopathy patients with KIF11 mutations and the Genotype-Phenotype correlation. Exp Eye Res. 2020;199:108165.
- ↑ Kondo H, Matsushita I, Nagata T, Fujihara E, Hosono K, Uchio E, et al. Retinal Features of Family Members with Familial Exudative Vitreoretinopathy Caused by Mutations in KIF11 Gene. Transl Vis Sci Technol. 2021;10(7):18.
- ↑ 24.0 24.1 24.2 Li J-K, Fei P, Li Y, Huang Q-J, Zhang Q, Zhang X, et al. Identification of novel KIF11 mutations in patients with familial exudative vitreoretinopathy and phenotypic analysis. Sci Rep. 2016:6:26564.
- ↑ 25.0 25.1 Hu H, Xiao X, Li S, Jia X, Guo X, Zhang Q. KIF11 mutations are a common cause of autosomal dominant familial exudative vitreoretinopathy. Br J Ophthalmol. 2016;100(2):278–83
- ↑ 26.0 26.1 26.2 26.3 26.4 Zhou Y, Xu M-F, Chen J, Zhang J-L, Wang X-Y, Huang M-H, et al. Loss-of-function of kinesin-5 KIF11 causes microcephaly, chorioretinopathy, and developmental disorders through chromosome instability and cell cycle arrest. Exp Cell Res. 2024;436(1):113975.
- ↑ 27.0 27.1 Maillard C, Roux CJ, Charbit-Henrion F, Steffann J, Laquerriere A, Quazza F, et al. Tubulin mutations in human neurodevelopmental disorders. Semin. Cell Dev. Biol. 2023;137
- ↑ 28.0 28.1 Thornton GK and Woods CG. Primary microcephaly: do all roads lead to Rome? Trends Genet. 2009;25:501-510.
- ↑ 29.0 29.1 Moritz M, Braunfeld MB, Sedat JW, Alberts B, and Agard DA. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature. 1995;378:638-640.
- ↑ Wieczorek M, Urnavicius L, Ti S-C, Molloy K-R, Chait BT, and Kapoor TM. Asymmetric Molecular Architecture of the Human γ-Tubulin Ring Complex. Cell. 2020;180(1):165-175.e16.
- ↑ 31.0 31.1 31.2 Bahtz R, Seidler J, Arnold M, Haselmann-Weiss U, Antony C, Lehmann WD, Hoffmann I. GCP6 is a substrate of Plk4 and required for centriole duplication. J Cell Sci. 2012;125(Pt 2):486-96.
- ↑ Thawani A, Petry S. Molecular insight into how γ-TuRC makes microtubules. J Cell Sci. 2021;134(14):jsc245464
- ↑ Miao H, Guo R, Chen J, Wang Q, Lee Y-R J, and Liu B. The γ-tubulin complex protein GPC6 is crucial for spindle morphogenesis but not essential for microtubule reorganization in Arabidopsis. Proc Natl Acad Sci USA. 2019;116(52):27115-27123.
- ↑ 34.0 34.1 34.2 34.3 Li Z, Li H, Xu X, Wang L, Liu B, Zheng W, et al. Haploinsufficiency of GCP4 induces autophagy and leads to photoreceptor degeneration due to defective spindle assembly in retina. Cell Death Differ. 2020;27(2):556-572.
- ↑ 35.0 35.1 35.2 35.3 35.4 Xu X, Shang D, Cheng H, Klionski DJ, Zhou R, et al. Gene essentiality of Tubgcp4: dosage effect and autophagy regulation in retinal photoreceptors. Autophagy. 2019;15(10):1834-1837.
- ↑ Dincer T, Yorgancioglu-Budak G, Olmez A, Er I, Dodurga Y, Ozdemir OM, et al. Analysis of centrosome and DNA damage response in PLK4 associated Seckel syndrome. European Journal of Human Genetics, 2017;25:1118-1125.
- ↑ Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543.
- ↑ Wang Y, Zhang Z, Huang L, Sun L, Li S, Zhang T, et al. Update on the Phenotypic and Genotypic Spectrum of KIF11-Related Retinopathy. Genes (Basel).2022;13(4):713.
- ↑ Wang Y, Smallwood PM, Williams J, Nathans J. A mouse model for kinesin family member 11 (KIF11) associated familial exudative vitreoretinopathy. Hum Mol Genet. 2020;29(7):1121-1131.