Aicardi Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Aicardi Syndome is a rare congenital disorder characterized by a classic triad of chorioretinal lacunae, infantile spasms, and agenesis of the corpus callosum. It is thought to be caused by an X-linked dominant mutation which is lethal to males in utero, though to date a causal gene has not yet been identified. [2][3][4][5]
Epidemiology
Incidence of Aicardi syndrome is 0.63 in 100,000 female births in Norway, 1 in 105,000-167,000 live births in the United States, and 1 in 100,000 live births in Northern Ireland. It is estimated that 4000 people worldwide are affected by Aicardi syndrome. There is no known association with ethnicity. [6][7][8]
Etiology
The etiology of Aicardi syndrome is thought to be an X-linked dominant mutation with hemizygous lethality in males. Many studies have explored potential genetic mechanisms, but no clear genetic abnormality has been identified.[9] Several reported cases of Aicardi syndrome in boys with an XXY genotype indicate an X-linked gene lethal in early development for XY fetuses.[9][10] Variability in disease manifestation suggests de novo pathogenic variants on the X-chromosome subject to X-chromosome inactivation.[11] More recent data suggests that the abnormal gene(s) at play may not actually be on the X-chromosome, but may instead be differentially expressed in males versus females or may be influenced by female sex hormones.[12]
Risk Factors
All known cases of Aicardi Syndrome are from de novo mutations with no familial inheritance pattern or identified risk factors.[9][10]
Diagnosis
History and Symptoms
The presenting symptom of Aicardi syndrome is usually infantile spasms, which can evolve into a variety of medically refractory seizure types.[13][14] There is variable psychomotor delay affecting mobility and language development. Skeletal malformations, especially costovertebral anomalies and scoliosis, are common.[11] Ocular manifestations include decreased visual acuity and vary with the degree of chorioretinopathy.[15][16]
Neurologic examination and findings
Brain imaging is characterized by partial to complete corpus callosum agenesis.[3][14] Recent studies have clarified other neuronal migration defects, most commonly cortical dysplasia, nodular heterotopias, and intracranial cysts.[9][17] Additional findings include posterior fossa abnormalities, basal ganglia dysmorphisms, polymicrogyria or pachygyria, gross cerebral asymmetry, and ventriculomegaly.[17][18][19] Cognitive development is delayed and most children have limited verbal communication and are unable to walk but can usually sit and feed themselves independently. Cognitive outcome is correlated with the severity of epilepsy, brain abnormalities, and ocular lesions.[15][16][20][21]
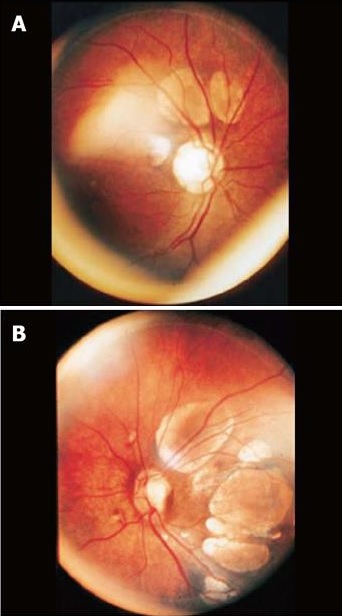
Ophthalmologic findings
Fundoscopic examination of patients with Aicardi Syndrome shows pathognomonic multiple chorioretinal lacunae that are typically bilateral, round, non-elevated, and without vessels crossing over their surface. Thinning of the choroid and sclera and degeneration of the overlying rods and cones causes a yellow-white to pinkish appearance. Variable border pigmentation can occur due to photoreceptor folds at the edges of the lesions that are lined with pigmented and nonpigmented epithelial cells. [18][3][15][16][19] The overlying retina is intact, but typically has an abnormal appearance on histology. Lacunae vary in size from 1/10th to 3 disc diameters and are clustered around the optic nerve head and posterior pole, decreasing in size towards the periphery. Lesion size and number are characteristically consistent over time, but some show increased or fading pigmentation.[3][15][16][20][8][19]
Additional ocular abnormalities include colobomas of the optic nerve and microphthalmos in approximately 44% and 20% of patients, respectively. Optic nerve anomalies, pupillary membrane remnants, iris colobomas, choroidal colobomas, morning glory discs, retinal detachment, altered foveal development, nystagmus, sixth cranial nerve palsy, glial tissue extending from the optic disc, hyperplastic primary vitreous, choroidal neovascularization, retinopathy of prematurity, severe congenital ptosis, late onset retinoblastoma, and aniridia have also been described in Aicardi Syndrome, albeit less frequently.[15][16][8][19]
Visual acuity is typically preserved if there is no foveal lacunar involvement, though low vision may be present if there is extensive foveal involvement and/or maldevelopment of the visual cortex. Optic disc and optic nerve anomalies are not predictive of visual function if there is good foveal development. [15][16]
Clinical diagnosis
Since no specific genetic mutation has thus far been identified, the diagnosis is clinical and may be made in the setting of the classic triad of agenesis of the corpus callosum, chorioretinal lacunae, and infantile spasms. Expansion of diagnostic criteria in 1999 includes patients with two features from the triad and at least two additional major or supporting features. [10][11][22]
Classic Triad
- Infantile spasms
- Chorioretinal lacunae
- Agenesis of the corpus callosum (partial or total)
Major Features
- Coloboma of the optic disc and nerve
- Cortical malformations (mainly microgyria)
- Periventricular (and subcortical) heterotopia
- Intracranial cysts: interhemispheric or around the third ventricle
- Papillomas of the choroid plexus
Supporting Features
- Vertebral and costal abnormalities
- Microphthalmia and/or other eye abnormalities
- ‘Split-brain’ EEG (dissociated suppression-burst tracing)
- Gross cerebral hemispheric asymmetry
- Vascular malformations or vascular malignancy
Differential diagnosis
Small or peripheral lacunae are associated with ocular toxoplasmosis, high myopia, orofacialdigital syndrome type IX and the syndrome of “microcephaly with present or absent chorioretinopathy, lymphedema, and mental retardation” (MCLMR). However, the lacunae in Aicardi syndrome have more defined borders and lack the additional features such as choriocapillaris sclerosis, fibrovascular proliferation, anomalies of Bruch’s membrane, fibrous metaplasia, or the presence of inflammatory cells that may be seen in other conditions in the differential. [3][23][24]
Management
General treatment
Treatment consists of management of infantile spasms with anti-epileptic medication and support for medically refractory epilepsy. Physical therapy, occupational therapy, speech therapy, and visual therapy should begin at the time of diagnosis to improve quality of life. In addition, supportive measures are often necessary for feeding difficulties and bowel dysfunction. Appropriate musculoskeletal support and treatment to prevent complications related to scoliosis is also indicated.[11][19]
Ophthalmologic treatment
There is currently no treatment for the visual abnormalities associated with Aicardi Syndrome, though some patients may benefit from low vision assistance. It is hypothesized that the association of peripheral retinal findings with optic disc anomalies may warrant early examination under anesthesia of the peripheral retina in patients with optic disc findings and laser therapy in the event of peripheral non-perfusion.[8]
Family planning
All reported cases are for a single affected family member, with the exception of cases in monozygotic twins. The recurrence risk for siblings is believed to be less than 1%, and there are no recorded cases of parent-to-child transmission. Corpus callosum agenesis on prenatal ultrasound or intrauterine MRI are suggestive but not diagnostic of a fetus with Aicardi Syndrome.[18][11][19]
Prognosis
If there is no foveal involvement, visual function can be normal. Visual function is typically poor in patients with foveal involvement and those with severe maldevelopment of the visual cortex, though visual manifestations are generally non-progressive. Psychomotor abilities vary in severity and appear to be correlated with lacunar size. Patients with larger lacunae are more likely to lack mobility and verbal or non-verbal language skills. [15][16][20][25] EEG (electroencephalogram) at the time of diagnosis may also help to predict long-term disease severity.[26] Most patients live into early adulthood; but those with severe epilepsy and organ involvement often die earlier due to aspiration pneumonia, systemic and respiratory infections, malignancy, and sudden death due to epilepsy.[25]
References
- ↑ Pires CR. Aicardi syndrome: Neonatal diagnosis by means of transfontanellar ultrasound. WJR. 2014;6(7):511. doi:10.4329/wjr.v6.i7.511
- ↑ Aicardi J, Chevrie JJ, Rousselie F. Spasma-in-flexion syndrome, callosal agenesis, chorioretinal abnormalities. Arch Fr Pediatr. 1969;26(10):1103-1120.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Fruhman G, Eble TN, Gambhir N, Sutton VR, Van den Veyver IB, Lewis RA. Ophthalmologic findings in Aicardi syndrome. J AAPOS. 2012;16(3):238-241. doi:10.1016/j.jaapos.2012.01.008
- ↑ Lund C, Striano P, Sorte HS, et al. Exome Sequencing Fails to Identify the Genetic Cause of Aicardi Syndrome. MSY. 2016;7(4):234-238. doi:10.1159/000448367
- ↑ Mavrommatis MA, Friedman AH, Fowkes ME, Hefti MM. Aicardi syndrome in a 20-year-old female. American Journal of Ophthalmology Case Reports. 2018;12:61-64. doi:10.1016/j.ajoc.2018.09.004
- ↑ Kroner BL, Preiss LR, Ardini M-A, Gaillard WD. New incidence, prevalence, and survival of Aicardi syndrome from 408 cases. J Child Neurol. 2008;23(5):531-535. doi:10.1177/0883073807309782
- ↑ Lund C, Bjørnvold M, Tuft M, Kostov H, Røsby O, Selmer KK. Aicardi syndrome: an epidemiologic and clinical study in Norway. Pediatr Neurol. 2015;52(2):182-186.e3. doi:10.1016/j.pediatrneurol.2014.10.022
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Shirley K, O’Keefe M, McKee S, McLoone E. A clinical study of Aicardi syndrome in Northern Ireland: the spectrum of ophthalmic findings. Eye (Lond). 2016;30(7):1011-1016. doi:10.1038/eye.2016.81
- ↑ Jump up to: 9.0 9.1 9.2 9.3 Wong BKY, Sutton VR. Aicardi syndrome, an unsolved mystery: Review of diagnostic features, previous attempts, and future opportunities for genetic examination. Am J Med Genet C Semin Med Genet. 2018;178(4):423-431. doi:10.1002/ajmg.c.31658
- ↑ Jump up to: 10.0 10.1 10.2 Aicardi J. Aicardi syndrome. Brain and Development. 2005;27(3):164-171. doi:10.1016/j.braindev.2003.11.011
- ↑ Jump up to: 11.0 11.1 11.2 11.3 11.4 Sutton VR, Van den Veyver IB. Aicardi Syndrome. 2006 Jun 30 [Updated 2020 Nov 12]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1381/
- ↑ Ha TT, Burgess R, Newman M, Moey C, Mandelstam SA, Gardner AE, Ivancevic AM, Pham D, Kumar R, Smith N, Patel C, Malone S, Ryan MM, Calvert S, van Eyk CL, Lardelli M, Berkovic SF, Leventer RJ, Richards LJ, Scheffer IE, Gecz J, Corbett MA. Aicardi Syndrome Is a Genetically Heterogeneous Disorder. Genes (Basel). 2023 Jul 31;14(8):1565.
- ↑ Glaze D. Etiology and pathogenesis of infantile spasms. UptoDate. Published online May 20, 2019. Accessed January 12, 2021. https://www.uptodate.com/contents/etiology-and-pathogenesis-of-infantile-spasms?sectionName=Inborn%20errors%20of%20metabolism&topicRef=2912&anchor=H14&source=see_link
- ↑ Jump up to: 14.0 14.1 Leeuw C de, Kurver A, Verrips A. Chorioretinal Lacunae in Aicardi’s Syndrome: Key for the Diagnosis. Neuropediatrics. 2020;51(4):311-312. doi:10.1055/s-0040-1709454
- ↑ Jump up to: 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Menezes AV, Enzenauer RW, Buncic JR. Aicardi syndrome--the elusive mild case. Br J Ophthalmol. 1994;78(6):494-496.
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 16.5 16.6 Menezes AV, Lewis TL, Buncic JR. Role of ocular involvement in the prediction of visual development and clinical prognosis in Aicardi syndrome. British Journal of Ophthalmology. 1996;80(9):805-811. doi:10.1136/bjo.80.9.805
- ↑ Jump up to: 17.0 17.1 Masnada S, Pichiecchio A, Formica M, et al. Basal ganglia dysmorphism in patients with Aicardi syndrome. Neurology. Published online December 4, 2020. doi:10.1212/WNL.0000000000011237
- ↑ Jump up to: 18.0 18.1 18.2 Beres S. Aicardi Syndrome. American Academy of Ophthalmology. Published September 2, 2020. Accessed February 2, 2021. https://www.aao.org/disease-review/neuro-ophthalmology-aicardi-syndrome
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 19.5 Zaarour K., Traboulsi, E. Aicardi Syndrome (AIC). American Academy of Ophthalmology. Published September 14, 2017. Accessed February 2, 2021. https://www.aao.org/disease-review/aicardi-syndrome-aic
- ↑ Jump up to: 20.0 20.1 20.2 Ospina LH, Nayak H, McCormick AQ. Progressive Pigmentation of Chorioretinal Lesions in Aicardi Syndrome. Archives of Ophthalmology. 2004;122(5):790-790. doi:10.1001/archopht.122.5.790
- ↑ Tuft M, Østby Y, Nakken KO, Lund C. Aicardi syndrome and cognitive abilities: A report of five cases. Epilepsy Behav. 2017;73:161-165. doi:10.1016/j.yebeh.2017.05.002
- ↑ Aicardi J. Aicardi Syndrome: Old and New Findings. Int Pediatr. 1999;14(1):5-8.
- ↑ McMahon RG, Bell RA, Moore GR, Ludwin SK. Aicardi’s syndrome. A clinicopathologic study. Arch Ophthalmol. 1984;102(2):250-253. doi:10.1001/archopht.1984.01040030200026
- ↑ Willis J, Rosman NP. The Aicardi syndrome versus congenital infection: Diagnostic considerations. The Journal of Pediatrics. 1980;96(2):235-239. doi:10.1016/S0022-3476(80)80808-0
- ↑ Jump up to: 25.0 25.1 Shah PK, Narendran V, Kalpana N. Aicardi syndrome: the importance of an ophthalmologist in its diagnosis. Indian J Ophthalmol. 2009;57(3):234-236. doi:10.4103/0301-4738.49403
- ↑ Masnada S, Alfei E, Formica M, Previtali R, Accorsi P, Arrigoni F, Bonanni P, Borgatti R, Darra F, Fusco C, De Giorgis V, Giordano L, La Briola F, Orcesi S, Osanni E, Parazzini C, Pinelli L, Rebessi E, Romaniello R, Romeo A, Spagnoli C, Uebler C, Varesio C, Viri M, Zucca C, Pichiecchio A, Veggiotti P. EEG at onset and MRI predict long-term clinical outcome in Aicardi syndrome. Clin Neurophysiol. 2022 Oct;142:112-124.