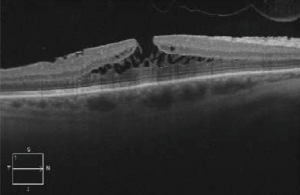
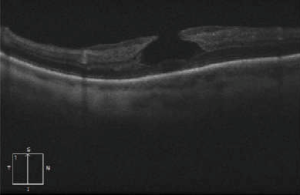
Lamellar Macular Hole
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Lamellar macular hole (LMH) is a retinal disorder that affects the macula involving a loss of tissue in the inner layers of the retina.
Etiology and Risk Factors
The majority of LMH cases are idiopathic. However, LMH has been linked to conditions causing cystoid macular edema (CME) (e.g., Irvine-Gass syndrome, diabetic macular edema, retinal vein occlusions[1]), as patients presenting such conditions may experience spontaneous rupture of the roof of a cystoid space. Conditions involving tractional forces applied to the retina, such as presence of tractitional epiretinal membranes (ERM) or a vitreomacular tractions (VMT), are also considered risk factors.
General Pathology
Though the understanding of LMH has evolved with new optical coherence tomography (OCT) findings, it remains unclear whether all LMH form through tractional forces exerted on the fovea or through degeneration of the inner layers of the retina. While initially described as a complication of CME, subsequent research identified conditions leading to the formation of LMH. Partial or complete posterior vitreous detachment (PVD) causing traction on the fovea can potentially lead to the formation of intraretinal cysts/schisis or ERM (i.e., contractile preretinal proliferation of myofibroblasts with an extracellular matrix). LMH may also be the consequence of an aborted FTMH formation process. On the other hand, certain LMH do not exhibit apparent signs of traction. Instead, they are rather associated to an epiretinal proliferation (ERP) of hyalocytes, fibroblasts, and glial cells lacking contractile properties and degeneration of the inner layers of the fovea; suggesting another subtype of LMH[2]. However, recent findings suggest that both the tractional and degenerative forms of LMH initially stem from a tractional force exerted onto retinal tissue[3][4].
Pathophysiology
Recent OCT studies[5] identified four pathways for the development of idiopathic LMH, all of which were tractional:
- Type 1: disruption of a foveal cyst from VMT;
- Type 2: rupture of parafoveal cysts or schisis mainly from ERM;
- Type 3: central intraretinal cyst formed under a tight ERM with subsequent cyst roof dehiscence;
- Type 4: gradual loss of foveal tissue without cystic lesions, initiated by ERM traction.
Indeed, both the “degenerative” and “tractional” phenotypes of LMH could begin with a tractional event that damages the Müller cells or its connections to the foveal walls, resulting in a schisis between the outer plexiform layer (OPL) and Henle fiber layer (HFL)[6]. Over time, this tractional event would cause gradual degeneration of Henle fibers, leading to the emergence and enlargement of the foveal cavitation. If combined with a secondary event, such as choroidal ischemia, this chronic HFL degeneration may cause loss of the retinal outer layers and ultimately lead to death of central photoreceptors and retrograde degradation of bipolar and horizontal cells[7]. Thus, the ERP that characterizes “degenerative” LMH could represent a reparation process resulting from an initial tractional insult.
If present, preretinal proliferation may correlate with visual acuity, with ERP leading to poorer visual acuity compared to ERM. Degenerative LMH configuration is also associated with higher rates of photoreceptor layer defects and lower residual foveal thickness[8][9][10].
Primary prevention
As most LMH cases are idiopathic, there are no preventative measures to stop the disease from occurring.
Diagnosis
History
LMH primarily affects individuals from 50–70 years of age, with an overall prevalence ranging from 1.1% to 3.6% in the general population according to population-based cohort studies[11][12].
Physical examination
Dilated fundus examination can indicate the presence of a macular hole, which can later be best confirmed as being lamellar with OCT or fundus autofluorescence[13].
Signs
OCT imaging showing the partial loss of inner foveal tissue is diagnostic. Fundus autofluorescence can also show hyperautofluorescence due to a window defect in the macular area from the loss of foveal tissue.
Symptoms
LMH symptoms, akin to other vitreoretinal interface syndromes, include reduced visual acuity, metamorphopsia, and central scotoma. While symptoms may be subtle initially, they tend to progress gradually[14].
Clinical diagnosis
OCT diagnostic criteria for LMH have undergone multiple changes over time. The most widely used 2006 criteria adopted by the International Vitreomacular Traction Study Group included:
- An irregular foveal contour;
- A break in the inner fovea;
- Dehiscence of the inner foveal retina from the outer retina;
- The absence of a full-thickness foveal defect with intact foveal photoreceptors[15][16].
Govetto et al. subsequently divided LMH into “tractional” and “degenerative” categories with a minimum of 3/5 diagnostic criteria for each type (see table)[4]. If it fails to correspond to either category, the lesion is classified as “mixed”.
| "Degenerative" LMH features | "Tractional" LMH features |
|---|---|
|
|
The newest consensus made by Hubschmann et al. distinguishes LMH presenting with tractional, contractile ERM by identifying them as ERM foveoschisis (ERMF)[16]. LMH presenting with degenerative configuration are simply referred to as LMH.
| Criteria | ERMF | LMH |
|---|---|---|
| Mandatory criteria |
|
|
| Optional anatomical features |
|
|
| OCT imaging |
This new classification suggests that LMH and ERM foveoschisis are two distinct entities. However, this definition may change as recent findings propose that both ERMF and LMH result from a tractional event; they would essentially represent the same condition at different stages of development[5].
Diagnostic procedures
OCT is the gold standard for diagnosing and managing LMH with findings as described above. Fundus autofluorescence can also be performed which shows hyperautofluorescence through loss of foveal tissue leading to a window defect.
Laboratory test
Laboratory investigations are not warranted for idiopathic LMH cases.
Differential diagnosis
Macular pseudohole (MPH)
MPH can have a very similar appearance to LMH. However, they differ in that, contrary to LMH, MPH does not involve loss of foveal tissue. The abnormal, hollow foveal contour found in MPH is rather due to contraction of an ERM against the foveal walls. Mandatory diagnostic criteria for MPH are ERM sparing foveal walls, retinal thickening, and verticalized or steepened foveal profile. Minor diagnostic criteria include near normal central foveal thickness[16]. These characteristics are not typical of LMH and can help differentiate the two conditions.
Vitreomacular traction (VMT)
VMT occurs during PVD when part of the vitreous remains attached to the retina, causing traction which can potentially damage the macula. This can lead to schisis of foveal tissue layers, which may mimic the aspect of LMH. Metamorphopsia can be experienced with both diseases. On OCT, VMT presents an attachment of the vitreous cortex to the macula and a distortion of the foveal surface. Unlike LMH, the layer of retinal tissue pulled by the vitreous is still connected to the rest of the fovea via its edges. However, VMT can lead to the formation of LMH.
Full-thickness macular hole (FTMH)
FTMH presents like LMH with loss of VA, metamorphopsia, and central scotoma. However, the lesion in this case is more severe than an LMH, with the fovea presenting a full-thickness defect. Stage 1 or stage 2 macular holes may be mistaken for LMH.
Management
General treatment
As most LMH remain stable over time, no treatment is indicated in idiopathic, asymptomatic cases. The treatment is surgical and consists of pars plana vitrectomy (PPV) which may involve the removal of preretinal tissue (ERM/ERP) and/or of the internal limiting membrane (ILM).
Medical therapy
As of today, no therapeutic modalities/drugs are indicated for the treatment of idiopathic LMH. Patients are simply monitored until spontaneous closure occurs in certain cases or symptoms develop warranting surgical management[17].
Medical follow up
Regular follow-up of LMH is essential to track the progression of the disease and consider surgical intervention if the LMH deteriorates over time, as they can progress to FTMH.
Surgery
Surgical treatment of LMH using PPV is usually indicated for patients with progressive deterioration of the foveal profile or symptomatic visual loss associated with metamorphopsia and/or central scotoma. It remains that the decision to intervene surgically is made on a case-by-case basis, weighing risks and benefits for the patient. PPV helps release vitreomacular adhesions by removing ERM/ERP and ILM, facilitating the restoration of a regular foveal profile. Tamponade with air or gas may be used, but longer duration gas is not critical for the surgery to succeed. In those cases, patients are usually instructed to maintain prone positioning postoperatively[18][19]. Removal of the ERM/ERP and ILM has been shown to induce closure of the LMH, notably by releasing tractional forces. However, peeling may be sometimes laborious, particularly with ERP which has a fluffy texture making it more difficult to peel[9]. Getting rid of the membrane around the LMH walls can lead to iatrogenic damage and the formation of FTMH postoperatively. Alternative techniques include the use of platelet rich plasma during surgery, the fovea-sparing ILM peel, as well as the embedding of the peeled ILM into the LMH. These approaches show promising results, as the articles in which they were described reported no cases of postoperative FTMH[20][21][22].
Surgical follow up
Regular surgical follow-ups are essential to assess for anatomical and functional success, as well as manage potential complications.
Complications
The most common surgical complication following PPV is cataract[1]. Non-closure of LMH and FTMH development are also reported[9][23][24][25]. Other complications include intraoperative retinal breaks, postoperative ocular hypertension or hypotension, and/or acute postoperative endophtalmitis[26].
Prognosis
Patients with LMH have a favorable prognosis, as most remain stable over time, with no significant visual acuity decline. While not necessary, surgical treatment is effective with most studies reporting a statistically significant improvement in visual acuity postoperatively[27].
References
- ↑ 1.0 1.1 Frisina R, Pilotto E, Midena E. Lamellar Macular Hole: State of the Art. Ophthalmic Res. 2019;61(2):73-82. doi:10.1159/000494687
- ↑ Denise Compera, Ricarda G Schumann, Matteo G Cereda, et al. Progression of lamellar hole-associated epiretinal proliferation and retinal changes during long-term follow-up. Br J Ophthalmol. 2018;102(1):84. doi:10.1136/bjophthalmol-2016-310128
- ↑ Wu L, Bradshaw R. Primary Lamellar Macular Holes: To Vit or Not to Vit. J Clin Med. 2022;11(17). doi:10.3390/jcm11175046
- ↑ 4.0 4.1 Govetto A, Dacquay Y, Farajzadeh M, et al. Lamellar Macular Hole: Two Distinct Clinical Entities? Am J Ophthalmol. 2016;164:99-109. doi:10.1016/j.ajo.2016.02.008
- ↑ 5.0 5.1 Lee CY, Hsia Y, Yang CM. Formation and evolution of idiopathic lamellar macular hole-a pilot study. BMC Ophthalmol. 2022;22(1):432. doi:10.1186/s12886-022-02669-4
- ↑ Bringmann A, Unterlauft JD, Wiedemann R, Rehak M, Wiedemann P. Morphology of partial-thickness macular defects: presumed roles of Müller cells and tissue layer interfaces of low mechanical stability. Int J Retina Vitr. 2020;6(1):28. doi:10.1186/s40942-020-00232-1
- ↑ Bringmann A, Unterlauft JD, Wiedemann R, Barth T, Rehak M, Wiedemann P. Degenerative lamellar macular holes: tractional development and morphological alterations. Int Ophthalmol. 2021;41(4):1203-1221. doi:10.1007/s10792-020-01674-0
- ↑ Pang CE, Spaide RF, Freund KB. EPIRETINAL PROLIFERATION SEEN IN ASSOCIATION WITH LAMELLAR MACULAR HOLES: A Distinct Clinical Entity. Retina. 2014;34(8):1513-1523. doi:https://doi.org/10.1097/IAE.0000000000000163
- ↑ 9.0 9.1 9.2 Parolini B, Schumann RG, Cereda MG, Haritoglou GP. Lamellar Macular Hole: A Clinicopathologic Correlation of Surgically Excised Epiretinal Membranes. Invest Ophthalmol Vis Sci. 2011;52(12):9074-9083. doi:https://doi-org./10.1167/iovs.11-8227
- ↑ Bottoni F, Deiro AP, Giani A, Orini C, Cigada M, Staurenghi G. The natural history of lamellar macular holes: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2013;251(2):467-475. doi:10.1007/s00417-012-2044-2
- ↑ Meuer SM, Myers CE, Klein BEK, et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the beaver dam eye study. Ophthalmology. 2015;122(4):787-795. doi:10.1016/j.ophtha.2014.10.014
- ↑ Liesenborghs I, De Clerck EEB, Berendschot TTJM, et al. Prevalence of optical coherence tomography detected vitreomacular interface disorders: The Maastricht Study. Acta Ophthalmol (Copenh). 2018;96(7):729-736. doi:10.1111/aos.13671
- ↑ Liu J, Zhao P. LAMELLAR MACULAR HOLE WITH LAMELLAR HOLE-ASSOCIATED EPIRETINAL PROLIFERATION IN FAMILIAL EXUDATIVE VITREORETINOPATHY. Retin Cases Brief Rep. 2021;15(4):365-368. doi:10.1097/ICB.0000000000000807
- ↑ Midena E, Vujosevic S. Metamorphopsia: An Overlooked Visual Symptom. Ophthalmic Res. 2015;55(1):26-36. doi:10.1159/000441033
- ↑ Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining Lamellar Holes and the Vitreomacular Interface: An Ultrahigh-Resolution Optical Coherence Tomography Study. Ophthalmology. 2006;113(3):388-397. doi:https://doi.org/10.1016/j.ophtha.2005.10.047.
- ↑ 16.0 16.1 16.2 Hubschman JP, Govetto A, Spaide RF, et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020;104(12):1741. doi:10.1136/bjophthalmol-2019-315432
- ↑ Catania F, Romano MR, Crincoli E, et al. Phenomenology of spontaneous closure in degenerative and mixed type lamellar macular hole. Eye Lond Engl. Published online July 31, 2023. doi:10.1038/s41433-023-02681-y
- ↑ Casparis H, Bovey EH. Surgical treatment of lamellar macular hole associated with epimacular membrane. Retina Phila Pa. 2011;31(9):1783-1790. doi:10.1097/IAE.0b013e31820a6818
- ↑ Garretson BR, Pollack JS, Ruby AJ, Drenser KA, Williams GA, Sarrafizadeh R. Vitrectomy for a symptomatic lamellar macular hole. Ophthalmology. 2008;115(5):884-886.e1. doi:10.1016/j.ophtha.2007.06.029
- ↑ Ho TC, Ho AYL, Chen MS. Reconstructing Foveola by Foveolar Internal Limiting Membrane Non-Peeling and Tissue Repositioning for Lamellar Hole-Related Epiretinal Proliferation. Sci Rep. 2019;9(1):16030. doi:10.1038/s41598-019-52447-4
- ↑ Frisina R, Tozzi L, Gius I, Pilotto E, Midena E. Novel approaches to the assessment and treatment of lamellar macular hole. Acta Ophthalmol (Copenh). 2022;100(6):e1287-e1297. doi:10.1111/aos.15078
- ↑ Kanai M, Sakimoto S, Takahashi S, et al. Embedding Technique versus Conventional Internal Limiting Membrane Peeling for Lamellar Macular Holes with Epiretinal Proliferation. Ophthalmol Retina. 2023;7(1):44-51. doi:10.1016/j.oret.2022.07.009
- ↑ Morescalchi F, Russo A, Gambicorti E, et al. PEELING OF THE INTERNAL LIMITING MEMBRANE WITH FOVEAL SPARING FOR TREATMENT OF DEGENERATIVE LAMELLAR MACULAR HOLE. Retina Phila Pa. 2020;40(6):1087-1093. doi:10.1097/IAE.0000000000002559
- ↑ Coassin M, Mastrofilippo V, Stewart JM, et al. Lamellar macular holes: surgical outcome of 106 patients with long-term follow-up. Graefes Arch Clin Exp Ophthalmol. 2018;256:1265-1273.
- ↑ Figueroa MS, Govetto A, Steel DH, Sebag J, Virgili G, Hubschman JP. PARS PLANA VITRECTOMY FOR THE TREATMENT OF TRACTIONAL AND DEGENERATIVE LAMELLAR MACULAR HOLES: Functional and Anatomical Results. Retina Phila Pa. 2019;39(11):2090-2098. doi:10.1097/IAE.0000000000002326
- ↑ Recchia FM, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS. Small-gauge pars plana vitrectomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2010;117(9):1851-1857. doi:10.1016/j.ophtha.2010.06.014
- ↑ Parisi G, Fallico M, Maugeri A, et al. Primary vitrectomy for degenerative and tractional lamellar macular holes: A systematic review and meta-analysis. PloS One. 2021;16(3):e0246667. doi:10.1371/journal.pone.0246667