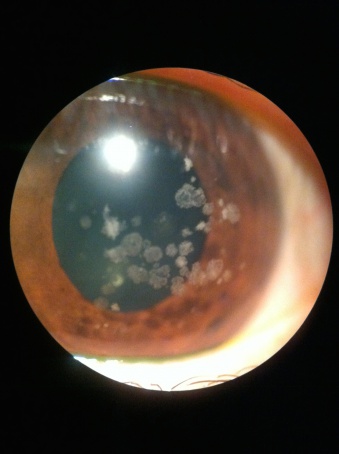
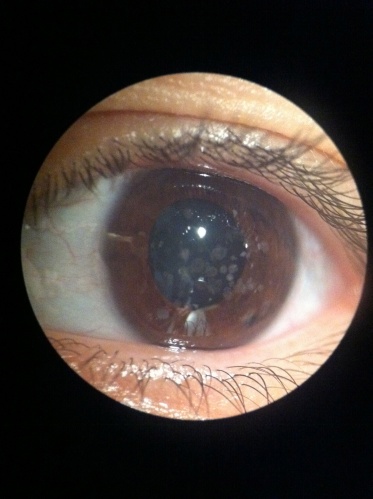
Corneal Stromal Dystrophies
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
The corneal dystrophies are a group of non-inflammatory, inherited, bilateral disorders of the cornea characterized by pathognomonic patterns of corneal deposition and morphological changes. The stromal subset of corneal dystrophies primarily affect the stroma, however, over time they often extend into the anterior corneal layers and some may affect Descemet membrane and the endothelium. Classically, the stromal corneal dystrophies have been classified based on their phenotypic appearance at the slit lamp and divided into the three main types of granular, lattice, and macular. As our understanding of the genetics of these dystrophies has expanded, a re-classification has occurred, as described by the International Committee for Classification of Corneal Dystrophies (IC3D). [1]
The morbidity associated with the stromal corneal dystrophies varies widely. If there is progressive accumulation of deposits in the cornea, the cornea may lose its clarity or the resulting uneven surface elevations may induce irregular astigmatism, and these effects will result in decreased vision for the patient. When stromal dystophies involve the anterior cornea, abnormal epithelial adhesion may lead to painful erosions.
Treatment options for the stromal dystrophies range depending on the symptoms. Lubricating drops and ointments can sometimes comfort a patient with foreign body sensation and may also help prevent painful erosions. Bandage contact lenses can be used to prevent and treat erosions. PTK may be attempted to remove anterior lesions. Ultimately, PK (penetrating keratoplasty) or DALK (deep anterior lamellar keratoplasty) can remove lesions and improve vision and comfort. However, the benefits of PK or DALK must be weighed against the risks of surgery and there is sometimes the risk of recurrence of the dystrophy within the new graft. Please refer to Eyewiki articles for individual dystrophies for a more detailed and individualized account of treatment options for each dystrophy.
The American Academy of Ophthalmology's Pathology Atlas contains virtual microscopy images of the following types of dystrophies:
Lattice Corneal Dystrophy Type 1
Also known as Biber-Haab-Dimmer corneal dystrophy, TGFBI type Lattice Dystrophy, or Classic Lattice Dystrophy.
Genetics & Inheritance
Autosomal dominant inheritance of the TGFBI gene on the 5q31 locus.
Slit Lamp Examination
Lattice dystrophy may be quite variable in its appearance, but it is most typically marked by 'glass-like' filamentous lesions. The deposits seen at the slit lamp may change over time, progressing from round, ovoid and white, or small, filamentous, and refractile anterior stromal lesions to more nodular, threadlike, and thicker linear lesions that extend into deep stroma. Typically, the limbus is not involved. While in the beginning stages there are clear spaces between lesions, over time these spaces opacify and take on a ground glass appearance. Signs of lattice dystrophy most often appear in early childhood and become more prominent into the 2nd and 3rd decades.
Pathology
Epithelium/Anterior Cornea:
- Atrophy and degeneration of basal epithelial cells
- Focal thinning or loss of Bowman layer
Stroma:
- Deposition of amyloid and abnormal corneal lamellar structure
Descemet membrane/Endothelium:
- Unaffected
Amyloid deposits stain with Congo red, PAS, Masson Trichrome, thioflavine-T fluorochrome, and are metachromatic with crystal violet. As with all amyloid, they demonstrate apple-green birefringence under polarized light.
On electron microscopy the deposits appear as electron-dense, randomly aligned fibrils, 8-10nm in diameter.
Symptoms
Symptoms of surface erosions, irregular astigmatism, and vision loss usually begin in the 2nd or 3rd decades of life.
The recurrent erosions in lattice dystrophy may be frequent. Some authors believe that phototherapeutic keratectomy should be avoided (as well as LASIK and PRK) because the excimer laser delivers energy within the UV spectrum, and this may trigger activation of TGFB to increase the number of lesions deposited.
Penetrating or lamellar keratoplasty may be necessary for patients with severe vision loss due to opacification (typically not until the 4th decade). Recurrence may occur in these grafts but presents differently than the primary lesions.
There are many subtypes of Lattice Corneal Dystrophy Type 1 and each varies in its age of onset, appearance, and rate of progression.
Lattice Corneal Dystrophy Type 2
Also known as Finnish Familial Amyloidosis, Meretoja syndrome, Amyloidosis V, Familial amyloidotic polyneuropathy IV
Genetics & Inheritance
Autosomal dominant inheritance of the Gelsolin gene on 9q34.
Slit Lamp Examination
As in lattice dystrophy type 1, filamentous lesions are present but they are more peripheral and less dense than in Type 1. The lesions tend to start at the limbus and progress inward. Most lesions are in the anterior stroma. The central cornea is less affected than in Type 1.
Associated ocular exam findings:
- Corneal hypoesthesia
- Dermatochalasis (due to amyloid deposition and secondary muscular dysfunction)
- Lagophthalmos
- Open angle glaucoma
Associated systemic findings:
- Dry, itchy skin
- Laxity of the facial skin
- Intermittent proteinuria (nephrotic syndromes)
- Cardiac conduction abnormalities, orthostatic hypotension, perspiration dysfunction
- Severe mask-like facial paresis with gradual onset of facial drooping, protruding lips and pendulous ears (due to amyloid deposition and secondary muscular dysfunction)
Pathology
Sub-Bowman layer deposits of amyloid lattice lines and intra-lamellar amyloid deposits, most prominent at the limbus.
The mutated gelsolin deposits can be found outside the cornea as well, in the conjunctiva and sclera, ocular nerves, extraocular arterial walls, peripheral nerves and the kidneys.
Symptoms
Unlike Type 1 Lattice dystrophy, in Type 2 vision is typically unaffected until middle age. Many patients will develop severe dry eye and corneal erosions later in life.
Granular Corneal Dystrophy Type 1
Also known as Groenouw type I corneal dystrophy.
Genetics & Inheritance
Autosomal dominant inheritance of the TGFBI gene on the 5q31 locus.
Slit Lamp Examination
Discrete crumb-like opacities are seen in the central anterior stroma. These deposits may appear in early childhood, and at first may appear as more subtle fine dots or lines before they develop into the more characteristic granules associated with this dystrophy. Early in life these granules are separated by clear cornea. The lesions do not typically extend to the limbus. They become more numerous over time. Late in the disease the lesions may coalesce and extend deep into the posterior stroma.
Homozygotes for the dystrophy have a more severe course and presentation.
Pathology
Hyaline deposits that stain bright red with Masson Trichome and are weakly PAS positive.
On electron microscopy (EM), the deposits appearance trapezoidal or rod-shaped.
Symptoms
While some visual symptoms such as glare and photophobia may occur early in life, visual acuity is not usually affected until the 4th decade for most patients. Recurrent erosions may occur. There is wide variety in patient symptoms.
Granular Corneal Dystrophy Type 2
Also known as Granular-Lattice Dystrophy, because it displays findings of both diseases, and Avellino Dystrophy, because it was first described in families from Avellino, Italy.
Genetics & Inheritance
Autosomal dominant inheritance of the TGFBI gene on the 5q31 locus.
Slit Lamp Examination
Deposits begin to appear in early childhood (in homozygotes) or adolescence as tiny whitish dots in the anterior stroma. Over time, these lesions progress into larger stellate, ring, or snowflake-like opacities. Lattice lines are seen later than the granular lesions and are usually found in deeper stroma. Some patients go on to develop almost confluent anterior granular lesions, but not as frequently as in type 1 granular.
Pathology
Mixed deposits of amyloid (as seen in Lattice dystrophy) and Hyaline (typical of Granular). The amyloid stains red with Congo red stain and the hyaline stains red with Masson Trichome.
Symptoms
Vision loss may occur earlier than in Type 1 Granular, with many patients noting vision loss in adolescence. However, few patients drop below the 20/70 level, even late in the disease.
Recurrent erosions and photophobia are also more common in Type 2 Granular than Type 1.
Macular Corneal Dystrophy
Also known as Groenouw type II corneal dystrophy or Fehr spotted dystrophy.
Genetics & Inheritance
Autosomal recessive inheritance of the carbohydrate sulfotransferase 6 gene (CHST) on 16q22.
Slit Lamp Examination
Stromal opacities with indistinct borders and intervening haze that may extend from limbus to limbus and through all layers of the cornea. While Descement's may be involved, the cornea is more often thin than thick or edematous.
Pathology
Accumulation of glycosaminoglycans in the cornea (stroma, Descemet membrane, endothelium) which stain with colloidal iron or Alcian blue.
The endothelium may display guttae as in Fuchs' dystrophy.
There are three variants of Macular dystrophy based on the immunoreactivity of the deposits--specifically, antigenic keratan sulfate:
- Type 1a: No reactivity of antigenic keratan sulfate in cornea or serum
- Type 1b: Reactivity intracellulary in the corneal stroma only
- Type 2: Reactivity of deposits in cornea (both intracellular and extracellular) with low or normal serum reactivity
Symptoms
Visual impairment, often severe, begins in childhood. Patients may also experience painful recurrent erosions or have loss of corneal sensation. Many complain of photophobia (light sensitivity).
Schnyder Corneal Dystrophy
Also known as Hereditary crystalline dystrophy of Schnyder, Schnyder corneal crystallline dystrophy, Crystalline stromal dystrophy, Central stromal crystalline corneal dystrophy.
Genetics & Inheritance
Autosomal dominant inheritance of the UbiA prenyltransferase domain containing 1 gene (UBIAD1) on 1p36.
Slit Lamp Examination
Initially, slit lamp exam reveals central haze and may display some crystals. By the 5th decade, many patients have haze throughout the entire cornea.
Only 50% of patients actually develop crystals. This is why the name was changed from Schnyder Crystalline Dystrophy to Schnyder Corneal Dystrophy.
Pathology
Phospholipid and cholesterol deposition in the stroma, Bowman layer and basal epithelium. These are best examined with special stains such as Sudan Black or Oil Red O.
Symptoms
Onset is typically made during the 2nd or 3rd decades of life. Patients develop increasing haze and difficulty in photopic more than scotopic conditions.
Congenital Stromal Corneal Dystrophy
Also known as Congenital hereditary stromal dystrophy and Congenital stromal dystrophy of the cornea.
Genetics & Inheritance
Autosomal dominant inheritance of the Decorin gene (DCN) on 12q21.33.
Slit Lamp Examination
Snowflake, whitish opacities appear throughout the entire cornea. Signs of inflammation are absent. Despite normal endothelium, the corneal thickness is often increased.
Pathology
The stromal lamellae are abnormal and may be separated by amorphous deposits. On transmission electron microscopy, collagen fibrils are approximately half of normal thickness.
Symptoms
Moderate to severe vision loss from birth due to corneal opacification. The degree of opacification may be static or slowly progressive.
Fleck Corneal Dystrophy
Also known as Francois-Neetens speckled corneal dystrophy.
Genetics & Inheritance
Autosomal dominant inheritance of the Phosphatidylinositol-3-phosphate/phosphatidylinositol 5-Kinase type III gene (PIP5K3) on 2q35.
Slit Lamp Examination
Reveals scattered small, discrete, grayish-white, dandruff-like opacities within an otherwise clear stroma.
Pathology
Swollen keratocytes with vacuoles containing glycosaminoglycans and lipid.
Symptoms
Asymptomatic
Some authors suggest including the following rare corneal dystrophies with the stromal corneal dystrophies: Gelatinous Drop-Like Corneal Dystrophy and Posterior Amorphous Corneal Dystrophy
1) Gelatinous Drop-Like Corneal Dystrophy: While this involves the anterior stroma, it is usually considered sub-epithelial and grouped with the superficial corneal dystrophies. Also known as Amyloid Corneal Dystrophy, and first described in 1914, this rare autosomal recessive condition is characterized by grayish-white corneal nodules. Most cases are reported from Japan and Southeast Asia.
2) Posterior Amorphous Corneal Dystrophy: This rare, autosomal dominant dystrophy, involves the posterior stroma as well Descemet membrane and even the iris. Slit lamp findings include large amorphous sheet-like opacifications of the posterior stroma and Descemet membrane, decreased corneal thickness, corneal flattening, scleralization of the peripheral cornea, iris coloboma, correctopia, iris atrophy, and iridocorneal adhesions.
Additional Resources
- Boyd K, Jimenez EM. Corneal Dystrophies. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/corneal-dystrophies-list. Accessed March 07, 2019.
- Feldman BH, Afshari NA. Corneal Dystrophies. Encyclopedia of the Eye. Ed. Dartt DA, Besharse JC, Dana R. Oxford: Elsevier Ltd, 2010. 416- 423.
- Emily S. Birkholz, MD, Nasreen A. Syed, MD, and Michael D. Wagoner, MD, PhD. Corneal Stromal Dystrophies: A Clinicopathologic Review. EyeRounds.Org. August 17, 2009 http://webeye.ophth.uiowa.edu/eyeforum/cases/43-Corneal-Stromal-Dystrophies.htm
- http://www.cornealdystrophyfoundation.org/
- http://www.emedicine.com/ophthalmology/index.shtml
- http://www.nei.nih.gov/health/cornealdisease/
References
- ↑ Weis JS et al. The IC3D Classification of the Corneal Dystrophies. Cornea.Cornea Volume 27, Suppl. 2, December 2008
- Afshari, N.A., Mullally, M.S., Afhsari M.A., et al. Survey of patients with Granular, Lattice, Avellino, and Reis-Bücklers corneal dystrophies for mutations in the BIGH3 and Gelsolin genes. Archives of Ophthalmology 119, 16-22.
- Afshari, N.A., Li Y.J., Pericak-Vance M.A, et al. Genome wide linkage scan in Fuchs endothelial corneal dystrophy. IOVS, 1-14.
- Bron, A.J. Genetics of the corneal dystrophies: What we have learned in the past twenty-five years. Cornea 19, 699-711.
- Dinh, R. Rapuano, C.J., Cohen E.J. et al. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology 106, 1490-97
- Holland EJ, Daya, S.M., Stone, E.M., et al. Avellino Corneal Dystrophy: clinical manifestations and natural history. Ophthalmology 99, 1564-1568.
- Kang P.C., Klintworth, G.K., Kim, T., et al. Trends in the indications for penetrating keratoplasty, 1980-2001. Cornea 24, 801-3.
- Krachmer, J.H., Mannis M.J., and Holland, E.J. ( 2005). Cornea (2nd Edition). Philadelphia: Elselvier Mosby.
- Kanski, J.J. (2003). Clinical Ophthalmology: A systematic approach. (5th Edition). Edinburgh: Butterworth Heinemannn.
- Ocular Pathology Atlas. American Academy of Ophthalmology Web site. https://www.aao.org/resident-course/pathology-atlas. Published 2016. Accessed January 10, 2017.
- Stone, E.M., Mathers, W.D., Rosenwasser, G.O., et al. Three autosomal dominant corneal dystrophies map to chromosome 5q. Nature Genetics 6, 46-51.
- Vasilliki, P., Colby K. Genetics of anterior and stromal corneal dystrophies. Seminars in Ophthalmomlogy 23, 9-17.
- Yanofff M., Duker J.S.. (2004). Ophthalmology. (2nd Edition). St. Louis: Mosby.
- Klintworth, Gordon K. “Corneal dystrophies.” Orphanet journal of rare diseases vol. 4 7. 23 Feb. 2009, doi:10.1186/1750-1172-4-7
- Carpel, Sigelman, Doughman, "Posterior Amorphous Corneal Dystrophy," American Journal of Ophthalmology, 30 Apr 1977, 83(5):629-632.