All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Branch retinal vein occlusion is the most common retinal vein occlusion.
Disease Entity
Branch retinal vein occlusion is recognized by the following codes by the International Classification of Diseases (ICD) nomenclature:
- 362.30 Retinal vein occlusion
- 362.35 Central retinal vein occlusion
- 362.36 Branch retinal vein occlusion
- 362.36 Tributary retinal vein occlusion
- 362.37 Incipient retinal vein occlusion
- 362.37 Partial retinal vein occlusion
- H34.83 Branch retinal vein occlusion
- H34.831 Branch retinal vein occlusion, right eye
- H34.832 Branch retinal vein occlusion, left eye
- H34.833 Branch retinal vein occlusion, bilateral
- H34.839 Branch retinal vein occlusion, unspecified eye

Disease
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder after diabetic retinopathy.[2] Retinal vein occlusions are divided into central retinal (CRVO), hemiretinal (HRVO), and branch retinal vein occlusions (BRVO). BRVO is a venous occlusion at any branch of the central retinal vein. Occlusion at the proximal part of the central retinal vein trunk results in an HRVO, which is considered a subtype of either CRVO or BRVO. The retina has a dual blood supply, with retinal vessels supplying the inner retina and choroidal vessels supplying the outer retina extending to the outer part of the inner nuclear layer.
Classification
BRVO is classified according to the anatomical location as major or macular. Major BRVO refers to occlusion of a retinal vein that drains one of the quadrants. Macular BRVO refers to occlusion of a venule within the macula. BRVO is most common in the superotemporal quadrant (58.1%-66%), followed by the inferotemporal quadrant (29%); it is least common in the nasal quadrants (12.9%).[3] [4] Macular BRVO involves the superior macular region in 81% of cases and the inferior macular region in 19% of cases.[5] The increased incidence in the superotemporal quadrant is thought to be due to increased arteriovenous crossings in that quadrant. BRVO is further classified as perfused (nonischemic) or nonperfused (ischemic). Ischemic BRVO is defined as >5 disc diameters of nonperfusion on fluorescein angiography (FA).[6] [7]
Epidemiology
BRVO is the most common RVO with an incidence of 0.44%-1.6%.[4][8] A meta-analysis completed in 2010 by Rogers et al pooled data from 11 studies from the United States, Europe, Asia, and Australia with 49,869 subjects and found that the estimated prevalence of any RVO was 0.52%, of BRVO was 0.442%, and of CRVO was 0.08%. Race, but not gender, was associated with higher prevalence. Overall, the prevalence of any RVO in increasing order by ethnicity was as follows: White, Black, Asian, and then Hispanic subjects. (The prevalence of BRVO was 0.282% in White, 0.353% in Black, 0.498% in Asian, and 0.598% in Hispanic subjects.)[8] Of BRVOs, 5%-10% are bilateral.
Risk Factors
BRVO has many known ophthalmic and systemic risk factors, including age, hypertension, hyperlipidemia, ocular hypertension, and glaucoma.[9][10] The incidence of BRVO increases with age with a prevalence of 0.157 per 100 in 40- to 49-year-olds, 0.458 per 100 in 50- to 59-year-olds, 1.11 per 100 in 60- to 69-year-olds, and 1.276 per 100 in 70- to 79-year-olds.[8]
After adjusting for age, the Beaver Dam Eye Study showed that BRVO was associated with hypertension, elevated systolic and diastolic pressure, pulse pressure, ocular perfusion, focal arteriolar narrowing, and arteriovenous nicking. After controlling for hypertension status, arteriovenous nicking and focal arteriolar narrowing remained as independent factors associated with BRVO.[4]
A large meta-analysis by O'Mahoney et al showed a significant association of hypertension and hyperlipidemia with BRVO. However, there was no association of diabetes mellitus with BRVO (unlike the association found with CRVO in the same study).[9][11] Other factors that are significantly associated with BRVO include glaucoma and body mass index (BMI).[9] [12] BRVO is uncommon in patients younger than 50 years, and there is a significant association with BMI in these patients.[13]
Various meta-analysis studies have examined the prevalence and association of thrombophilic risk factors with BRVO. The only significant associations are hyperhomocysteinemia and anticardiolipin antibodies with BRVO. However, controversy remains about an association with MTHFR gene mutation, factor V Leiden mutation, protein C and S deficiency, prothrombin gene mutation, or antithrombin deficiency. Anticardiolipin antibodies can be found in antiphospholipid syndrome, systemic lupus erythematosus, and Behcet disease.[12][14][15]
Summary of risk factors:
- Increasing age
- Ocular hypertension/glaucoma
- Hypertension
- Hyperlipidemia
- Body mass index
- Hyperhomocysteinemia
- Anticardiolipin antibodies
Pathogenesis
The pathogenesis of BRVO is multifactorial in origin and not completely defined. Possible mechanisms include a combination of mechanical compression, degenerative changes in vessel walls, and/or hypercoagulable factors. The arteriosclerotic changes (specifically arteriovenous crossing) are believed to result in venule occlusion through endothelial cell damage and thrombosis. Another hypothesis is that arteriosclerosis results in arteriolar insufficiency leading to BRVO.
The association between BRVO and arteriovenous crossings has been established in multiple studies. In almost all cases of BRVO (97.6%-100%),[16][17][18] the thick-walled artery is found anterior to the thin-walled vein. The artery and vein also share a common adventitial sheath at these crossings, which contributes to the predisposition of vein occlusion. Arteriolar sclerosis increases the rigidity of the artery and further provides support for the mechanical basis of BRVO at arteriovenous crossings.[16][19] Mechanical compression of the vein by the rigid artery results in turbulent blood flow at arteriovenous crossings, resulting in venous intima media and endothelial damage, which leads to vein occlusion.[3][20] Virchow triad[21] consists of 3 important factors for vascular thrombosis: hypercoagulability, abnormal blood flow, and endothelial injury.
In histological studies of BRVO, the endothelium and intima media are found to be thickened and altered at the arteriovenous crossings, with no blood thrombus obliterating the venous lumen at the arteriovenous crossing, which suggests compression is a major factor in the pathogenesis of BRVO. Postmortem and postenucleation histological studies of chronic BRVO have shown the thickening of the common adventitial tissue of the artery and vein at the arteriovenous crossings.[22]
Recent use of optical coherence tomography (OCT) has shown that retinal veins exhibit luminal narrowing at arteriovenous crossings, rather than compression or flattening of the venous lumen, which might seem contradictory to the compressive mechanism of BRVO. However, there is a degree of venous narrowing correlated with the diameter of the crossing artery. Arteries that are larger due to arteriosclerosis result in veins with narrower lumens at arteriovenous crossing. The narrowed veins retain their circular shape in cross-section. The narrowed veins have to change their course at the arteriovenous crossing, resulting in venous tortuosity that extends beyond the external limiting membrane line on OCT. The change in the course of the vein results in turbulent blood flow, which causes chronic damage to the venous endothelial cells, leading to endothelial cell proliferation and venous wall modeling. The endothelial cell dysfunction with narrowing of the venous lumen contributes to BRVOs.[23]
The higher incidence of superotemporal BRVO compared to other quadrants is speculated to be due to a larger number of arteriovenous crossings in that quadrant, which supports the hypothesis of arteriovenous nicking as a cause. The Eye Disease Case-Control Study Group showed that patients with BRVO had significantly more arteriovenous crossings than subjects matched for age, sex, and race. Also, of the 106 eyes with BRVO, 105 had the artery located anterior to the vein at the site of blockage.[3]
Macular edema is the main cause of vision loss in BRVO. The pathogenesis of macular edema is believed to be a result of multiple inflammatory cascades. Analysis of vitreous samples from patients with BRVO has established an association with increased levels of vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), IL-8, and monocyte chemoattractant protein 1 (MCP-1), compared to controls.[24][25][26] Excess VEGF is produced from retinal epithelial cells, endothelial cells, and Müller cells in the setting of BRVO, resulting in vascular permeability and contributing to macular edema.[27]
Diagnosis
BRVO is a clinical diagnosis that is further classified with OCT and FA.
History/Symptoms
Patients presenting with BRVO have a range of symptoms depending on the site and severity of the occlusion. Typically, patients present with sudden painless vision loss or visual field defect. Rarely, patients present with floaters from vitreous hemorrhage if the initial vein occlusion was asymptomatic and retinal neovascularization had developed.
Physical Examination/Signs
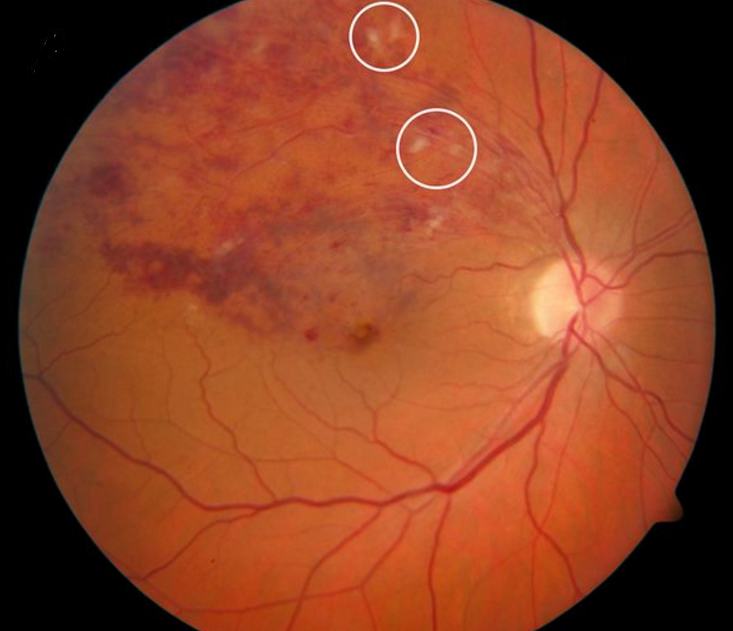
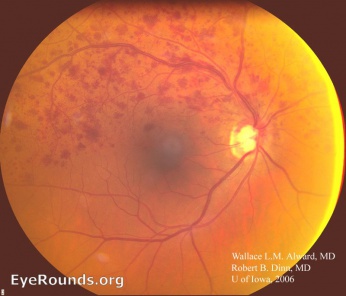
Typical funduscopic examination consists of flame hemorrhages, dot-and-blot hemorrhages, cotton-wool spots, hard exudates, retinal edema, and dilated tortuous veins (Figure 1). Classically there is a wedge-shaped distribution of intraretinal hemorrhage that is more extensive if the occlusion is ischemic compared to a nonischemic BRVO. A hemorrhage can be present at the arteriovenous crossing, which is called the Bonnet sign.[29] The Bonnet sign in hypertensive retinopathy denotes banking (dilatation) of the vein distal to the arteriovenous crossing.
Patients can present with visual field defects. Patients with macular BRVO present with a central field defect, while those with major BRVO present with a peripheral field defect corresponding to the retinal quadrant that is affected. The field defects are relative in cases of capillary nonperfusion and can become absolute scotomas in long-standing occlusions.
In patients with chronic BRVO, the intraretinal hemorrhages are absorbed and subsequent retinal vascular abnormalities develop in the distribution of the BRVO. The resulting changes include capillary nonperfusion, collateral formation, microaneurysms, sclerosed veins, and telangiectatic vessels (Figure 2).
Rarely, patients with undiagnosed BRVO or nasal BRVO can present with neovascularization of the iris, angle, and/or posterior segment. Signs and symptoms may include vitreous hemorrhage, rubeosis, and pain from neovascular glaucoma.
Clinical Diagnosis
Patients with BRVO should have a complete history and ophthalmic examination, with particular attention to the history of glaucoma, intraocular inflammation, and systemic risk factors for BRVO. Careful examination of the undilated iris and angle should be performed on initial and subsequent examinations to monitor for early signs of rubeosis and neovascular glaucoma.
Cause of Visual Decline
Visual decline in BRVO may be caused by the following:
- Macular edema
- Macular ischemia
- Hemorrhage over the fovea
- Vitreous hemorrhage from neovascularization of the retina or the optic disc
- Epiretinal membrane/vitreomacular traction
- Tractional retinal detachment/combined tractional and rhegmatogenous retinal detachment/rhegmatogenous retinal detachment
Diagnostic Procedures
Fluorescein Angiography
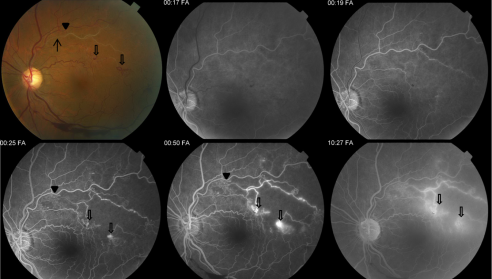
In patients with BRVO, fluorescein angiography helps to characterize the retinal vasculature, including the extent of nonperfusion, macular ischemia, macular edema, and leakage. However, on initial presentation, intraretinal hemorrhages may make obtaining a quality FA difficult. The various retinal abnormalities on OCT and FA have prognostic significance and help identify complications due to BRVO.
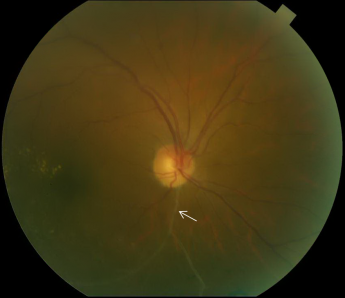
The characteristic FA for BRVO shows delayed filling of the occluded retinal vein with varying degrees of capillary nonperfusion. Intraretinal hemorrhages will result in blockage, and macular edema or retinal neovascularization will result in dye extravasation (Figure 3).
Optical Coherence Tomography
OCT allows for rapid, noninvasive, quantitative analysis of the macula and has become an important imaging modality in the assessment and treatment of BRVO. OCT has been used in many studies as the primary imaging modality in preference to FA, since assessment and treatment most often focus on macular edema.
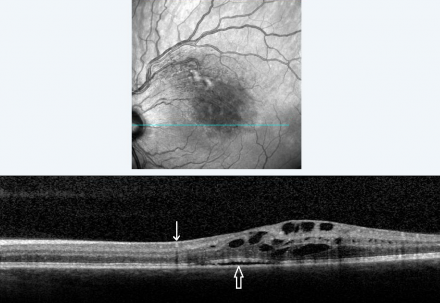
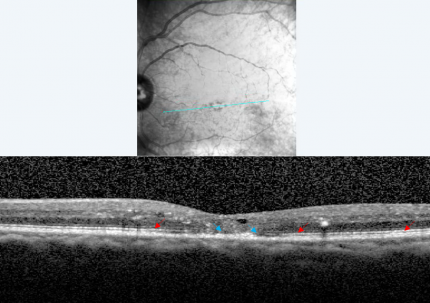
Characteristic findings of BRVO on OCT are cystoid macular edema, intraretinal hyperreflectivity from hemorrhages, shadowing from edema and hemorrhages, and occasionally subretinal fluid (Figure 4). Unlike FA, OCT images are minimally affected by intraretinal hemorrhages. The third high reflectance band corresponds to the junction between inner segments (IS) and outer segments (OS) of photoreceptors. This band (also called the ellipsoid zone) has prognostic significance for visual acuity. Disruption or absence of the ellipsoid zone after macular edema resolution is an indication of photoreceptor cell death or disarrangement and correlates with poor visual acuity (Figure 5).[30]
Laboratory Test
The diagnosis of BRVO is based on clinical examination. Typical patients will present with a history of known risk factors (hypertension, hyperlipidemia, or glaucoma). Initial workup includes a detailed history to define risk factors. Initial laboratory workup consists of a comprehensive metabolic panel, complete blood count, and lipid panel to help optimize known risk factors in consultation with the primary care physician.
In patients younger than 50 years, with bilateral BRVO at presentation, or with a history of numerous BRVOs, a search for an infectious, inflammatory, or hypercoagulable cause is warranted. Initial workup includes a detailed history with attention to other risk factors that might predispose to a hypercoagulable state, including medications (such as oral contraceptives). The workup should be performed in consultation with the primary care doctor and/or hematologist. Laboratory workup should include a complete metabolic panel, complete blood count, prothrombin time/partial thromboplastin time, vitamin B6/B12 and folate levels, homocysteine level (hyperhomocysteinemia), antiphospholipid antibodies (lupus anticoagulant, anticardiolipin antibody), protein C and S levels (deficiency), antithrombin levels (deficiency), and activated protein C resistance (factor V Leiden).
Inflammatory BRVO is clinically identified with evidence of vasculitis and intraocular inflammation (paravascular infiltrates, vitritis, snowballs). The blockage of the vein in such cases does not necessarily correspond to the arteriovenous crossing. These BRVOs are usually peripheral and multiple, and the vasculitis may be in different stages (active, healing, or healed) in the same eye.
Differential Diagnosis
- Hypertensive retinopathy
- Diabetic retinopathy
- Radiation retinopathy
- Macular telangiectasia
- Retinal angiomatous proliferation
- Age-related macular degeneration or variants such as retinal angiomatous proliferation
- Ocular contusion or retinal trauma
Management
The treatment of BRVO is aimed at the complications that cause vision loss, including macular edema, macular ischemia, and neovascularization. Systemic risk factors should be optimized in consultation with the primary care doctor. Anticoagulation has no proven benefit in the management of BRVO associated with a coagulopathy.[31]
Laser Photocoagulation
Prior to the advent of anti-VEGF agents, laser photocoagulation was considered the gold standard for the treatment of BRVO with macular edema or retinal neovascularization as established by the Branch Vein Occlusion Study (BVOS) Group.[6][7] Photocoagulation can be considered in patients with perfused macular edema with visual acuity (VA) ≤20/40 without improvement in VA for at least 3 months. Before application of laser photocoagulation, an FA of the macula is obtained to delineate the affected area of capillary nonperfusion and source of leakage as well as to determine if visual loss is due to macular edema or ischemia with an enlarged foveal avascular zone.
The recommended parameters for grid laser include duration of 0.1 seconds, spot size diameter of 100 µm, and power to produce a medium white burn. FA may be repeated in 2-4 months to assess the need for additional laser and resolution of macular edema. Laser photocoagulation is applied in a grid pattern throughout a leaking area but not involving the edge of the capillary-free zone or beyond the major vascular arcades peripherally.
The mechanism of action of laser photocoagulation in macular edema is thought to be destruction of nearby capillary beds, reducing input of arterial blood supply and allowing remaining capillaries to drain into less-congested, intact capillary beds.
Laser photocoagulation is also used to prevent vitreous hemorrhage. According to results from the BVOS Group, patients with BRVO and >5 disc diameters of retinal capillary nonperfusion should undergo scatter laser photocoagulation only once neovascularization has developed. BVOS data showed that scatter photocoagulation after neovascularization develops is as effective as performing it before neovascularization develops in preventing vitreous hemorrhage.
The recommended parameters for scatter laser include duration of 0.1-0.2 seconds, spot size diameter of 200-500 µm, and power to produce a medium white burn. Scatter laser is applied in the area of capillary nonperfusion as defined by FA, with laser applied peripherally at least to the equator and no closer than 2 disc diameters from the center of the fovea.
Steroid Treatment
Corticosteroids have been shown to be effective for the treatment of macular edema in BRVO.[32] However, intraocular corticosteroids have significant side effects including progression of cataract formation and elevation of intraocular pressure (IOP). The SCORE-BRVO study is the largest trial that evaluated the safety and efficacy of intravitreal triamcinolone compared to grid laser in the treatment of macular edema.[33] At 12 months, the study concluded that there was no difference in visual acuity gained between the 2 triamcinolone groups and grid laser group. There was significant cataract formation and elevation of IOP in the intravitreal triamcinolone groups. The 3-year results showed a significant increase in vision in the laser group compared to the intravitreal triamcinolone groups. From the results of this study, intravitreal triamcinolone is not recommended as a first-line therapy for macular edema due to BRVO. It is utilized as an adjunct to laser or anti-VEGF agents or as a second-line agent.
Ozurdex, a dexamethasone implant, was evaluated in the GENEVA study for macular edema in CRVO and BRVO. The implant is injected using a 22-gauge custom injector and gradually releases dexamethasone over several months (1-3 months), with peak response at 60 days. The study found that the Ozurdex implant group had a higher percentage of patients gaining ≥15 letters at the 90-day period compared to the sham group and also a lower percentage of patients losing ≥15 letters. However, the study is limited by the use of a sham treatment rather than laser treatment in the control group, particularly for BRVO eyes where laser has been shown to be of benefit.[34]
Anti-VEGF Agents
In patients with BRVO, retinal ischemia results in elevated secretion of VEGF, leading to increased vascular permeability and vasodilation.[25][26][27] There are several anti-VEGF agents available to treat macular edema due to BRVO, including ranibizumab (Lucentis), bevacizumab (Avastin), aflibercept (Eylea), and faricimab (Vabysmo).
Ranibizumab, aflibercept, and faricimab are approved by the US Food and Drug Administration (FDA) for the treatment of macular edema following RVO. Ranibizumab is a recombinant humanized monoclonal antibody fragment (Fab) that binds all VEGF-A isoforms at higher affinity than bevacizumab. Bevacizumab is a full-length humanized monoclonal antibody that binds all VEGF-A isoforms and is used off-label in the eye. Aflibercept (VEGF-trap) is a recombinant fusion protein that consists of human VEGF receptor 1 and 2 extracellular domains fused to the Fc portion of human IgG1. Aflibercept binds to all forms of VEGF-A with higher affinity than bevacizumab and ranibizumab and also targets VEGF-B and placental growth factor (PlGF).[35] Faricimab is a humanized bispecific IgG monoclonal antibody that binds and inhibits both VEGF and angiopoietin 2 (Ang-2).
Ranibizumab (Lucentis)
In the BRAVO study, patients with macular edema due to BRVO were randomized to monthly 0.5-mg ranibizumab, 0.3-mg ranibizumab, or sham injections for the first 6 months (treatment period). Patients were allowed 1 rescue grid laser treatment during the treatment period and were screened for rescue grid laser at 3 months. If they had a gain of <5 ETDRS letters or <50-µm improvement in central subfield thickness they qualified for rescue grid laser. If rescue laser was not used at the 3-month time point, they were further screened monthly for possible rescue laser.
At the 6-month time point, eyes in the 0.3-mg and 0.5-mg ranibizumab groups gained a mean of 16.6 and 18.3 letters, respectively. There was a 7.3 letter gain in the sham group with 54.5% of the sham group requiring rescue laser. Only 18.7% and 19.8% of the 0.3-mg and 0.5-mg ranibizumab groups, respectively, required rescue laser.
After the first 6 months, all groups were allowed as-needed (PRN) ranibizumab (observation period). The qualification for PRN ranibizumab was vision ≤20/40 or mean central subfield thickness ≥250 µm. Patients were again allowed 1 rescue grid laser treatment starting at 9 months. At 12 months, the mean letter gain was 16.4 and 18.3 in the 0.3-mg and 0.5-mg groups, and 12.1 in the sham/0.5-mg group.
The BRAVO study showed that ranibizumab is superior to traditional grid laser for macular edema from BRVO. The study also showed that PRN treatment was able to maintain gains in vision after 5 monthly injections. However, the study did not evaluate when to switch to a PRN schedule.
The HORIZON trial was a 12-month extension of the BRAVO and CRUISE trials and followed patients every 3 months for 12 months. Patients were given PRN ranibizumab 0.5 mg based on the criteria in the BRAVO study. Overall, HORIZON concluded that PRN use of ranibizumab after an intensive year of treatment was able to maintain visual acuity gains.[36][37][38]
Aflibercept (Eylea)
The VIBRANT study compared intravitreal aflibercept 2 mg to grid laser. Patients with macular edema due to BRVO that occurred within 12 months with best-corrected visual acuity (BCVA) 20/40 to 20/320 were included in the trial.
Patients were randomized to aflibercept drug injections every 4 weeks for the first 20 weeks or grid laser at baseline followed by a single rescue grid laser from weeks 12 through 20. After 24 weeks, the drug injection group received intravitreal aflibercept injection every 8 weeks, while the grid laser group received sham injections. Patient in the grid laser group who qualified for rescue laser were given 3 monthly drug injections followed by drug injections every 8 weeks. Rescue treatment criteria included >50-µm increase in central subfield thickness compared with lowest previous measurements, loss of ≥5 letters compared with best previous measurement in conjunction with any increase in central subfield thickness, or presence of new or persistent cystic retinal changes, subretinal fluid, or persistent diffuse edema.
At 24 weeks, the mean change in BCVA was 17.0 in the aflibercept group compared to 6.9 in the grid laser group. At 52 weeks, the mean change in BCVA was 17.1 in the aflibercept group compared to 12.2 in the grid laser group. Rescue treatment was required in 10% of the drug group and in 80% of eyes in the laser/drug group.
Overall, the VIBRANT study showed that aflibercept is more effective than grid laser in the treatment of macular edema from BRVO, and the vision gains established after monthly injections can be maintained by extending treatment.
The VIBRANT study included patients with perfused and nonperfused BRVO, unlike the BRAVO study, which only included perfused BRVO. The VIBRANT study evaluated retinal perfusion at baseline, 24 weeks, and 52 weeks. It showed an improvement in retinal perfusion and reversal of nonperfusion after treatment with intravitreal aflibercept. This effect was also noted in the laser/drug group at 52 weeks after they received the drug from weeks 24 to 52, but not during the laser-only portion of the study.[36][39][40]
Bevacizumab (Avastin)
There are numerous small prospective studies evaluating 1.25-mg bevacizumab in the treatment of macular edema due to BRVO. One of the larger studies by Russo et al assigned 30 eyes to either grid laser or intravitreal bevacizumab. Bevacizumab was given at 1, 3, 6, and 12 months. Grid laser was applied at baseline and reassessed at 3 months for possible retreatment. At 12 months, the mean gain in BCVA was 0.31 logMAR (15 letters) in the bevacizumab group compared with 0.20 logMAR (10 letters) in the grid laser group.[41] The mean gain in this study is similar to mean gains in the BRAVO/HORIZON and VIBRANT studies.
The dose of bevacizumab was evaluated in a retrospective study by Wu et al, where 2.5-mg and 1.25-mg bevacizumab were compared. There was no statistically significant difference between the 2 doses on BCVA and central subfield thickness. The mean BCVA gain at 6 months was 5.1 lines and 4.5 lines in the 1.25-mg group and 2.5-mg group, respectively.[42]
The BERVOLT study retrospectively looked at the efficacy and safety of bevacizumab in macular edema due to BRVO and CRVO. It concluded that the overall mean change in BCVA in the BRVO group treated with 1.25-mg bevacizumab was 0.25 logMAR (13 letters) with no significant adverse events.[43]
Faricimab (Vabysmo)
The phase 3 BALATON trial evaluated the efficacy, safety, and durability of faricimab in patients with macular edema due to BRVO.[44] Patients were randomized to 6 monthly injections of faricimab 6 mg or aflibercept 2 mg. From weeks 24-72, all patients received faricimab administered at up to 16-week intervals using a personalized treatment interval algorithm (treat and extend). The primary endpoint was noninferiority of faricimab versus aflibercept in mean change from baseline in BCVA at week 24. Secondary endpoints included treatment durability at week 68. A total of 553 patients were enrolled in BALATON. At week 24, the study met its endpoint of noninferiority with a +16.9 letter gain in the faricimab arm versus a +17.5 letter gain in the aflibercept arm.[45]
Surgical Management
Sheathotomy
Most venous obstructions in BRVO occur at an arteriovenous crossing. In histopathologic studies in BRVO, arteries and veins share a common adventitial sheath. The first case report for pars plana vitrectomy combined with sheathotomy for BRVO was done by Osterloh et al. The patient improved from 20/200 to 20/25+ over an 8-month period.[46] Since then, there have been various case series assessing surgical treatment options for BRVO.
Mester et al published a case series of 43 patients with macular edema due to BRVO treated with arteriovenous decompression and isovolemic hemodilution compared to 25 patients who underwent isovolemic hemodilution only after 6 weeks. Sixteen of the 43 patients also underwent internal limiting membrane removal in the macular area, besides the arteriovenous dissection. At 6 weeks, the mean BCVA improved by 0.19 logMAR (10 letters) in the arteriovenous decompression group compared with no change in mean BCVA in the isovolemic hemodilution group.[47]
Muqit et al published a study on long-term prospective visual outcomes of arteriovenous sheathotomy with or without intravitreal triamcinolone. The study included 8 patients of which 3 received intravitreal triamcinolone. The mean follow-up was 34.5 months with no overall improvement in BCVA; however, there was improvement in vascular perfusion.[48]
The published literature is inconclusive on the benefits of surgical management of macular edema due to BRVO. Another possible mechanism for improvements in visual acuity has been hypothesized to be related to increased oxygenation of the macula after vitrectomy. Overall, this modality remains controversial and has fallen out of favor as a treatment method by most retinal surgeons.
Vitrectomy
In cases where neovascular complications occur such as nonclearing vitreous hemorrhage, pars plana vitrectomy may be considered, usually in combination with intraoperative endolaser to the nonperfused portion of the retina affected by the BRVO. Some retinal surgeons also consider pars plana vitrectomy with internal limiting membrane peeling for treatment of recalcitrant RVO-associated macular edema, but this remains controversial.
Isovolemic Hemodilution
Chen et al published the first results of isovolemic hemodilution in patients with BRVO and decreased visual acuity with hematocrit ≥35%. Patients randomized to treatment with volume replacement using hydroxyethylstarch were compared to untreated patients. At 1-year follow-up, the final VA was 20/40 in the treated group compared with 20/80 in the untreated group.[49]
Since then, further studies evaluating various agents for hemodilution have shown a consistent VA improvement of 2 lines. However, there are many reported systemic complications due to hemodilution, including headaches, exertional dyspnea, fatigue, deep vein thrombosis, and hypotension. Due to the systemic invasiveness of the treatment and the many systemic complications, isovolemic hemodilution is not generally accepted as a treatment for BRVO.[19]
Medical Follow-up
The major complications that result in vision loss in BRVO include macular edema, macular ischemia, and neovascularization. The typical follow-up should be tailored on an individual basis to monitor for the development of these complications. After initial presentation, the typical follow-up should be every 1-2 months to monitor for the development of macular edema and/or neovascularization. If macular edema develops, treatment with anti-VEGF therapy with or without laser should be initiated and monitored for resolution. Once edema has resolved or stabilized, the follow-up interval can be gradually extended to 3 to 6 months or longer for stable chronic cases. Patients with nonperfused BRVO (>5 disc diameters) who have not been treated with laser should be monitored every 3 months due to increased risk of neovascularization.
Complications
The most common cause of decreased vision in BRVO is macular edema. Other complications include ischemic maculopathy, retinal neovascularization, macroaneurysmal formation, retinal telangiectasia, retinal detachment, and vitreous hemorrhage.
Macular edema occurs in 90% of BRVO cases. Reduced blood flow to the macula results in increased upregulation of endothelin 1 (ET-1), inflammatory cytokines, and VEGF, which results in increased endothelial permeability leading to macular edema and exudates. Macular edema can be divided into perfused and nonperfused, with better VA prognosis for perfused macular edema.[24][50][51]
Vitreous hemorrhage occurs in 3.9% of BRVO cases.[52] Chronic retinal nonperfusion results in increased levels of VEGF, which stimulates neovascularization. Disc hemorrhages are less common in BRVO but have been noted in normal-tension glaucoma patients with BRVO.[53] Neovascular glaucoma rarely occurs in BRVO cases.[54]
Serous retinal detachment occurs in up to 20% of BRVO cases, and it is more common in major BRVO than in macular BRVO.[50] Retinal breaks occur in 3% of BRVO cases and rhegmatogenous retinal detachment occurs in 1.3% of BRVO cases.[55]
Prognosis
BRVO has a generally good visual prognosis with 50%-60% of patients having final visual acuity ≥20/40 even without treatment. The natural course of BRVO depends on the type of occlusion, degree of nonperfusion, and development of collaterals. Poor prognostic indicators are chronic macular edema, macular BRVO, and neovascularization resulting in vitreous hemorrhage.[5][54][56][57]
In untreated symptomatic BRVO, the initial VA ranges from 20/40 to less than 20/200. VA improves in 1/3 to 3/4 of patients by at least 2 lines. On average, VA improves by 1 letter at 3 months to 15 letters at 18 months in untreated eyes. In patients presenting with macular edema, on average about 18% resolve without treatment by 4.5 months, and 41% resolve by 7.5 months.[58]
Patients presenting with 20/200 or better VA have higher rates of final VA ≥20/200 (33%-83%) regardless of whether they receive treatment. Eyes with initial VA worse than 20/50 without treatment have a poorer prognosis with a lower chance of final VA ≥20/200 (0-25%). Patients with initial VA ≥20/50 without treatment have a better prognosis with the majority of eyes with final VA ≥20/50 (56%-90%).[19] However, even though there is improvement in VA without treatment, significant visual acuity improvement beyond 20/40 is uncommon.[58]
To clarify the natural prognosis of BRVO without treatment, Hayreh et al reviewed 216 cases of BRVO (consisting of major and macular BRVO) that were followed for a median of 3 years. After resolution of macular edema, 76% of eyes with major BRVO and 58% of eyes with macular BRVO had improvement by ≥3 lines on Snellen chart. The median time to resolution of BRVO, consisting of resolution of both macular edema and retinal hemorrhages, is 21 months in major BRVO and 18 months in macular BRVO.[58]
On initial presentation, temporal major BRVO presents with VA ≥20/60 in 51% of cases, with no difference between superior or inferior temporal arcade involvement. In macular BRVO, initial VA is ≥20/60 in 74% of cases, with no difference between inferior and superior macular BRVO. In major BRVO with initial VA ≥20/60, 75% improve (gain of 3 lines on Snellen chart) or remain stable. Of eyes with initial VA ≤20/70, 69% improve by 3 lines on Snellen chart.[5]
Developing BRVO in 1 eye increases the risk of BRVO in the fellow eye to 7%-10%.[6][7][54][58][59] The Beaver Dam Eye Study showed no association of increased all-cause mortality or ischemic heart disease death after controlling for age in subjects with BRVO.[4]
Summary of Studies
BVOS—1984 and 1986[6][7]
- Objective: to assess if photocoagulation can (1) prevent neovascularization, (2) prevent vitreous hemorrhage, and (3) improve vision in eyes with macular edema with ≤20/40 from BRVO
- Total patients: 319 + 82 +139
- Duration: mean of 3.1 years
- Groups:
- Eyes with ≥5 disc diameters of nonperfusion without neovascularization (NV) treated with either scatter laser or no laser
- Eyes with NV treated with either scatter laser or no laser
- Eyes with BRVO and macular edema and visual acuity ≤20/40 randomized to grid laser or no laser
- Results:
- 319 eyes recruited: 12% of treated eyes and 22% of nontreated eyes developed NV. However, there was no change in visual acuity from baseline.
- 82 eyes recruited: 29% of treated eyes and 61% of nontreated eyes developed vitreous hemorrhage (VH); 12% of eyes that developed VH had vision loss.
- 139 eyes recruited: 65% of treated eyes and 37% of nontreated eyes gained 2 or more lines from baseline. Overall, treated eyes gained 1.33 lines of vision and nontreated eyes gained 0.23 lines.
- Conclusion: Eyes randomized to grid laser had a visual acuity gain.
The study was not powered to determine if laser treatment should be applied before or after NV to prevent VH. The BVOS Group recommended the use of grid laser for BRVO with macular edema and VA ≤20/40. It also recommended following nonperfused eyes at 4-month intervals and the use of scatter laser once NV develops.
SCORE-BRVO—2009[33]
- Objective: to compare the efficacy and safety of 1-mg and 4-mg doses of preservative-free intravitreal triamcinolone with standard care (grid laser)
- Total patients: 411 with macular edema due to BRVO
- Duration: 12 months
- Randomization to 1-mg or 4-mg preservative-free intravitreal triamcinolone or macular grid laser
- Results: All 3 groups had an equivalent percentage of patients with a gain of ≥15 letters from baseline at 12 months. The rates of elevated IOP and cataracts were similar between the grid laser and 1-mg groups; however, both adverse effects were significantly higher in the 4-mg group. Of note, the volume of all injections was 0.05 mL.
- Summary: There is no difference in visual acuity gained at 12 months between grid laser and triamcinolone; however, there are higher rates of cataract formation and IOP elevation with the use of triamcinolone 4 mg.
GENEVA—2010[34]
- Objective: to evaluate the efficacy and safety of a dexamethasone intravitreal implant (DEX implant, Ozurdex) in CRVO and BRVO with macular edema
- Total patients: 830 with BRVO and macular edema for 6 weeks to 12 months, and 437 with CRVO and macular edema for 6 weeks to 9 months
- Duration: 6 months
- Randomization to sham procedure, 0.35-mg DEX implant, or 0.7-mg DEX implant
- Results were not separated by BRVO and CRVO and were analyzed as a single group:
- At 30-90 days, the DEX implant groups had a higher percentage of eyes with BCVA gain ≥15 letters.
- A lower percentage of eyes had a loss of ≥15 letters in the DEX implant groups compared to sham group at all time points.
- Summary: DEX implants can improve the speed of visual improvement in eyes with macular edema due to BRVO or CRVO. The study was limited by the absence of rescue laser for treatment in the sham group.
BRAVO—2011[36][37]
- Objective: to evaluate the efficacy and safety of ranibizumab in the treatment of macular edema following BRVO
- Total patients recruited: 397 with BRVO and macular edema diagnosed within 12 months
- Duration: 12 months
- Randomization to ranibizumab 0.3 mg, ranibizumab 0.5 mg, and sham/ranibizumab 0.5 mg (with ranibizumab administered during the observation period based on specific criteria)
- Protocol: The treatment period (first 6 months) consisted of monthly injections with possible rescue laser at 3 months based on specific criteria. The observation period (second 6 months) consisted of monthly assessments with PRN ranibizumab if vision ≤20/40 or mean central foveal thickness ≥250 µm. Grid laser photocoagulation was allowed once at 3 and 9 months if specific criteria were met.
- Results: At 6 months, the mean letter gain was 16.6 and 18.3 in the 0.3-mg and 0.5-mg ranibizumab groups and 7.3 in the sham group. Of the sham group, 54.5% received rescue laser, compared with 18.7% and 19.8% in the 0.3-mg and 0.5-mg ranibizumab groups. At 12 months, the mean letter gain was 16.4 and 18.3 in the 0.3-mg and 0.5-mg groups, compared with 12.1 in the sham/0.5-mg group.
- Summary: Ranibizumab treatment showed better vision improvement than grid laser at 6 months. As-needed ranibizumab is able to maintain the benefits achieved after monthly scheduled injections of ranibizumab for macular edema from BRVO.
HORIZON—2012[38]
- Objective: to evaluate the efficacy and safety of ranibizumab in RVO patients
- Total patients: 304 with BRVO and 304 with CRVO from BRAVO and CRUISE trials, respectively
- Duration: every 3 months for 12 months
- Patients were recruited for this extension study from prior trials (BRAVO and CRUISE) and monitored every 3 months, receiving ranibizumab 0.5 mg if they met prespecified criteria.
- Results for BRVO subjects: The number of injections in the 0.3/0.5-mg and 0.5-mg ranibizumab groups and the sham/0.5-mg group was 2.4, 2.1, and 2.0, respectively. The mean BCVA gains achieved at month 12 of the BRAVO study were reduced by 2.0 (0.3/0.5 mg) and 1.7 (0.5 mg) and increased by 2.4 (sham/0.5 mg). In this 12-month period, 9%-13% of BRVO patients required rescue laser.
- Summary: Despite the reduced frequency of injections in the second year after the BRAVO study, patients were able to cement their gains.
VIBRANT—2015[39][40]
- Total patients: 181 eyes with either BRVO or HRVO with foveal center-involved macular edema
- Duration: every 4 weeks for 52 weeks
- Patients were randomized to intravitreal aflibercept injections (IAI) every 4 weeks for the first 20 weeks or grid laser at baseline followed by a single rescue grid laser from weeks 12 through 20. After 24 weeks, the IAI group received IAI every 8 weeks, while the grid laser group received sham injections. Patients in the grid laser group who qualified for rescue laser were given 3 monthly IAI followed by IAI every 8 weeks. The IAI group was assessed for rescue laser at 36 weeks and given grid laser if qualified.
- Rescue treatment criteria included >50-µm increase in central subfield thickness compared with lowest previous measurements, loss of ≥5 letters compared with best previous measurement in conjunction with any increase in central subfield thickness, or presence of new or persistent cystic retinal changes, subretinal fluid, or persistent diffuse edema.
- Results: At 24 weeks, the mean change in BCVA was 17.0 in the IAI group, compared with 6.9 in the grid laser group. At 52 weeks, the mean change in BCVA was 17.1 in the IAI group, compared with 12.2 in the grid laser group. In the IAI group, 10% of eyes received rescue laser, and in the laser/IAI group, 80% of eyes received rescue IAI. At baseline, both groups had a similar percentage of nonperfused BRVO. At 24 weeks, there was a significant reduction in the number of nonperfused BRVO in the IAI group compared to the laser group. At 52 weeks, there was no difference in nonperfused BRVO between the 2 groups.
- Summary: Monthly IAI for 6 months followed by IAI every 8 weeks resulted in maintained control of macular edema and sustained improvement in BCVA for 52 weeks.
BERVOLT—2015[43]
- Total patients: 87 eyes with BRVO
- Duration: mean follow-up of 24.4 months
- Retrospective, noncomparative case series of BRVO patients who received ≥1 intravitreal injection of bevacizumab
- Results: There was significant improvement of visual acuity with a mean change in BCVA of 0.25 logMAR (13 letters) at 2 years with an average of 7.6 injections.
- Summary: Bevacizumab is an alternative to other anti-VEGF therapies for BRVO with macular edema. The letter gain equivalent is around 15, which is similar to findings in the BRAVO/HORIZON studies. However, the study was limited by the retrospective protocol, lack of randomization, 14-month delay between diagnosis and treatment, and variable protocol use of bevacizumab.
Other Studies
RELATE
- Title: Ranibizumab Dose Comparison (0.5 mg and 2.0 mg) and the Role of Laser in the Management of Retinal Vein Occlusion: A Pharmacodynamic Approach
- Patients were randomized to 0.5-mg or 2-mg ranibizumab every 4 weeks for 24 weeks and re-randomized to PRN ranibizumab plus laser or ranibizumab alone. Patients with CRVO and BRVO were included.
- No short-term clinically significant benefit was found with 2-mg versus 0.5-mg ranibizumab injections. In addition, there was no long-term benefit in BCVA, resolution of edema, or number of ranibizumab injections needed with the addition of laser treatment to ranibizumab.[60]
BRIGHTER
- Title: Efficacy and Safety of Ranibizumab With or Without Laser in Comparison to Laser in Branch Retinal Vein Occlusion
- In 2016, the BRIGHTER study showed that ranibizumab with or without laser is better than laser alone. Visual acuity results were superior for the ranibizumab-treated arms of the study compared to the laser arm, with a 14.8 letter gain for the ranibizumab arms versus 6.0 for the laser arm at 6 months (primary endpoint).[61]
- At 24 months, the ranibizumab arms continued to do better than the laser arm. The addition of laser did not decrease the number of anti-VEGF injections. No new safety signals were noted.[62]
Additional Resources
- Boyd K. What is branch retinal vein occlusion (BRVO)? American Academy of Ophthalmology. EyeSmart/Eye Health. October 1, 2024. Accessed April 7, 2025. https://www.aao.org/eye-health/diseases/what-is-branch-retinal-vein-occlusion
- Porter D. Blood pressure. American Academy of Ophthalmology. EyeSmart/Eye Health. February 27, 2018. Accessed April 7, 2025. https://www.aao.org/eye-health/anatomy/blood-pressure
References
- ↑ Behrman S. Richard Liebreich, 1830-1917. First iconographer of the fundus oculi. Br J Ophthalmol. 1968;52(4):335-338.
- ↑ Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33(5):901-910.
- ↑ Jump up to: 3.0 3.1 3.2 Zhao J, Sastry SM, Sperduto RD, Chew EY, Remaley NA. Arteriovenous crossing patterns in branch retinal vein occlusion. The Eye Disease Case-Control Study Group. Ophthalmology. 1993;100(3):423-428.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133-141.
- ↑ Jump up to: 5.0 5.1 5.2 Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retina Eye Res. 2014;41:1-25.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984;98(3):271-282.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Branch Vein Occlusion Study Group. Arch Ophthalmol. 1986;104(1):34-41.
- ↑ Jump up to: 8.0 8.1 8.2 Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313-319.e1.
- ↑ Jump up to: 9.0 9.1 9.2 Risk factors for branch retinal vein occlusion. The Eye Disease Case-Control Study Group. Am J Ophthalmol. 1993;116(3):286-296.
- ↑ Kolar P. Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J Ophthalmol. 2014;2014:724780.
- ↑ O'Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126(5):692-699.
- ↑ Jump up to: 12.0 12.1 Yau JW, Lee P, Wong TY, Best J, Jenkins A. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904-910.
- ↑ Lam HD, Lahey JM, Kearney JJ, Ng RR, Lehmer JM, Tanaka SC. Young patients with branch retinal vein occlusion: a review of 60 cases. Retina. 2010;30:1520-1523.
- ↑ Ajith TA, Ranimenon. Homocysteine in ocular diseases. Clin Chim Acta. 2015;450:316-321.
- ↑ Janssen MC, den Heijer M, Cruysberg JR, Wollersheim H, Bredie SJ. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93(6):1021-1026.
- ↑ Jump up to: 16.0 16.1 Duker JS, Brown GC. Anterior location of the crossing artery in branch retinal vein obstruction. Arch Ophthalmol. 1989;107(7):998-1000.
- ↑ Christoffersen NL, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. 1999;106:2054-2062.
- ↑ Weinberg D, Dodwell DG, Fern SA. Anatomy of arteriovenous crossings in branch retinal vein occlusion. Am J Ophthalmol. 1990;109:298-302.
- ↑ Jump up to: 19.0 19.1 19.2 Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33:111-131.
- ↑ Jefferies P, Clemett R, Day T. An anatomical study of retinal arteriovenous crossings and their role in the pathogenesis of retinal branch vein occlusions. Aust N Z J Ophthalmol. 1993;21:213-217.
- ↑ Tripathy K, Sharma YR, Chawla R, Basu K, Vohra R, Venkatesh P. Triads in ophthalmology: a comprehensive review. Semin Ophthalmol. 2017;32(2):237-250. doi:10.3109/08820538.2015.1045150
- ↑ Frangieh GT, Green WR, Barraquer-Somers E, Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol. 1982;100(7):1132-1140.
- ↑ Kumagai K, Tsujikawa A, Muraoka Y, et al. Three-dimensional optical coherence tomography evaluation of vascular changes at arteriovenous crossings. Invest Ophthalmol Vis Sci. 2014;55:1867-1875.
- ↑ Jump up to: 24.0 24.1 Fraenkl SA, Mozaffarieh M, Flammer J. Retinal vein occlusions: the potential impact of a dysregulation of the retinal veins. EPMA J. 2010;1:253-261.
- ↑ Jump up to: 25.0 25.1 Yoshimura T, Sonoda K, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4(12):e8158.
- ↑ Jump up to: 26.0 26.1 Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005;140(2):256-261.
- ↑ Jump up to: 27.0 27.1 Karia N. Retinal vein occlusion: pathophysiology and treatment options. Clin Ophthalmol. 2010;4:809-816.
- ↑ Dinn R, Alward W. BRVO (branched retinal vein occlusion). EyeRounds.org. February 8, 2008. Accessed April 7, 2025. https://webeye.ophth.uiowa.edu/eyeforum/atlas/pages/BRVO-Branched-Retinal-Vein-Occlusion.html
- ↑ Kimmel AS, Magargal LE, Morrison DL, Robb-Doyle E. Temporal branch retinal vein obstruction masquerading as a retinal arterial macroaneurysm: the Bonet sign. Ann Ophthalmol. 1989;21:251-252.
- ↑ Ota M, Tsujikawa A, Murakami T, et al. Association between integrity of foveal photoreceptor layer and visual acuity in branch retinal vein occlusion. Br J Ophthalmol. 2007;91:1644-1649.
- ↑ Chatziralli IP, Jaulim A, Peponis VG, Mitropoulos PG, Moschos MM. Branch retinal vein occlusion: treatment modalities: an update of the literature. Semin Ophthalmol. 2014;29(2):85-107. doi:10.3109/08820538.2013.833271
- ↑ McAllister IL, Vijayasekaran S, Chen SD, Yu D-Y. Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol. 2009;147:838-846.
- ↑ Jump up to: 33.0 33.1 The SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115-1128.
- ↑ Jump up to: 34.0 34.1 Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134-1146.e3.
- ↑ Sharma YR, Tripathy K, Venkatesh P, Gogia V. Aflibercept--how does it compare with other anti-VEGF drugs? Austin J Clin Ophthalmol. 2014;1(3):1016.
- ↑ Jump up to: 36.0 36.1 36.2 Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102-1112.e1.
- ↑ Jump up to: 37.0 37.1 Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594-1602.
- ↑ Jump up to: 38.0 38.1 Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119:802-809.
- ↑ Jump up to: 39.0 39.1 Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015;122:538-544.
- ↑ Jump up to: 40.0 40.1 Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016;123(2):330-336.
- ↑ Russo V, Barone A, Conte E, Prascina F, Stella A, Noci ND. Bevacizumab compared with macular laser grid photocoagulation for cystoid macular edema in branch retinal vein occlusion. Retina. 2009;29:511-515.
- ↑ Wu L, Arevalo JF, Roca JA, et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008;28(2):212-219.
- ↑ Jump up to: 43.0 43.1 Kornhauser T, Schwartz R, Goldstein M, Neudorfer M, Loewenstein A, Barak A. Bevacizumab treatment of macular edema in CRVO and BRVO: long-term follow-up. (BERVOLT study: Bevacizumab for RVO long-term follow-up). Graefes Arch Clin Exp Ophthalmol. 2016;254(5):835-844.
- ↑ Hattenbach LO, Abreu F, Arrisi P, et al. BALATON and COMINO: phase III randomized clinical trials of faricimab for retinal vein occlusion: study design and rationale. Ophthalmol Sci. 2023;3(3):100302. doi:10.1016/j.xops.2023.100302
- ↑ Tadayoni R, Paris LP, Danzig CJ, et al. Efficacy and safety of faricimab for macular edema due to retinal vein occlusion: 24-week results from the BALATON and COMINO trials. Ophthalmology. 2024;131(8):950-960. doi:10.1016/j.ophtha.2024.01.029
- ↑ Osterloh MD, Charles S. Surgical decompression of branch retinal vein occlusions. Arch Ophthalmol. 1988;106(10):1469-1471.
- ↑ Mester U, Dillinger P. Vitrectomy with arteriovenous decompression and internal limiting membrane dissection in branch retinal vein occlusion. Retina. 2002;22:740-746.
- ↑ Muqit MM, Saidkasimova S, Keating D, Murdoch JR. Long-term study of vascular perfusion effects following arteriovenous sheathotomy for branch retinal vein occlusion. Acta Ophthalmol. 2010;88(3):e57-e65.
- ↑ Chen HC, Wiek J, Gupta A, Luckie A, Kohner EM. Effect of isovolaemic haemodilution on visual outcome in branch retinal vein occlusion. Br J Ophthalmol. 1998;82(2):162-167.
- ↑ Jump up to: 50.0 50.1 Yamaguchi Y, Otani T, Kishi S. Serous macular detachment in branch retinal vein occlusion. Retina. 2006;26:1029-1033.
- ↑ Finkelstein D. Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol. 1992;110:1427-1434.
- ↑ Lee YJ, Kim JH, Ko MK. Neovascularization in branch retinal vein occlusion combined with arterial insufficiency. Korean J Ophthalmol. 2005;19:34-39.
- ↑ Yoo YC, Park KH. Disc hemorrhages in patients with both normal tension glaucoma and branch retinal vein occlusion in different eyes. Korean J Ophthalmol. 2007;21:222-227.
- ↑ Jump up to: 54.0 54.1 54.2 Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488-506.
- ↑ Kir E, Saatci AO, Ozbek Z, Kaynak S, Ergin MH. Retinal breaks and rhegmatogenous retinal detachment in association with branch retinal vein occlusion. Ophthalmic Surg Lasers. 1999;30:285-288.
- ↑ Finkelstein D. Argon laser photocoagulation for macular edema in branch vein occlusion. Ophthalmology. 1986;93(7):975-977.
- ↑ Hayreh SS, Zimmerman MB. Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol. 2014;132(1):13-22.
- ↑ Jump up to: 58.0 58.1 58.2 58.3 Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094-1101.e5.
- ↑ Michels RG, Gass JD. The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol. 1974;78(2):OP166-OP177.
- ↑ Campochiaro PA, Hafiz G, Mir TA, et al. Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: the RELATE trial. Ophthalmology. 2015;122(7):1426-1437.
- ↑ Tadayoni R, Waldstein SM, Boscia F, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology. 2016;123(6):1332-1344.
- ↑ Tadayoni R, Waldstein SM, Boscia F, et al. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-month results of the BRIGHTER study. Ophthalmology. 2017;124(12):1778-1787.