Usher Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
2018 ICD-10 Code: H35.53: Other dystrophies primarily involving the sensory retina.
Disease
Usher syndrome, also known as Hallgren syndrome, is a rare genetic condition that is characterized by progressive vision and hearing loss.
Usher syndrome has been classified into three major subtypes: I, II, and III.
While all three types involve progressive vision loss due to retinitis pigmentosa (RP), they are categorized according to the genes responsible and the onset and severity of the signs and symptoms.
Usher Syndrome Type I is the most severe subtype. It is characterized by profound congenital hearing loss, RP, and absent vestibular function.
Usher Syndrome Type II is less severe. Patients with this subtype have moderate-to-severe congenital hearing loss, RP, and normal vestibular function.
Usher Syndrome Type III involves progressive hearing loss, RP, and varying degrees of vestibular dysfunction. Onset for type III is typically within the second to fourth decades of life. These patients also tend to have better vision than the other subtypes.[1]
Genetics
The mode of inheritance for Usher Syndrome is autosomal recessive. 13 genes and 16 loci have been found to contribute to Usher Syndrome.[2]
Usher Syndrome I Genes: CDH23, MYO7A, PCDH15, USH1C, CIB2 and USH1G
Usher Syndrome II Genes: USH2A, GPR8, and DFNB31.
Usher Syndrome III Genes: CLRN1 and PDZD7
Usher Syndrome I Loci: USH1B, USH1C, USH1D, USH1E, USH1F, USH1G, USH1H, USH1J, USH1K
Usher Syndrome II Loci: USH2A, USH2C, USH2D
Usher Syndrome III Loci: USH3A
In general, genes associated with Usher syndrome provide instructions for the synthesis of proteins involved in normal hearing, balance, and vision.[3]
Epidemiology
Usher syndrome is a rare disease in the general population with a prevalence of 3 cases per 100,000 in the population.[4] It is significantly more prevalent the genetically deaf accounting for approximately 5-10% of those with the handicap.[5] In the United States it occurs in approximately 1 in 23,000 people.[6] It has been found that Type I Usher Syndrome is more common among people of Ashkenazi Jewish or French Acadient descent.[7][8] Type III accounts for only about 2% of all cases. It has been shown that Type III occurs more frequently in the Finnish population.[9]
Pathophysiology
Hearing loss is due to a defect in the inner ear hair cells. Mutations in the USH genes result in defects of the corresponding proteins that are components in properly forming stereocilia bundles and growths. These defects cause deficits in hearing and vestibular function. Additionally, transmission of neuronal signals from inner ear hair cells is potentially compromised by USH mutations.[2]
Vision loss results from retinitis pigmentosa (RP) which involves the degeneration of retinal cells. Research on the exact mechanism of vision loss from Usher Syndrome is ongoing, but current literature revolves around the interface of the inner and outer segments of photoreceptors. At the junction of the inner and outer segments are connecting cilium and the periciliary ridge region, which form the periciliary membrane complex (PMC). This complex serves an essential role as a diffusion barrier controlling transport to the outer segment. Current theories propose that defects in the USH1 protein complex (myosin VIIa, harmonin, cadherin-23, protocadherin-15 and sans) leads to disruption in the organization of the PMC.[10] Furthermore, mutations in USH2 will interfere with the USH2 proteins (usherin, ADGVR1, and whirlin) in the PMC, which typically provide structural support between the PMC and connecting cilium. Additionally, Myosin VIIa, coded by MYO7a, has a direct involvement in transporting melanosomes and phagosomes in retinal pigment epithelium (RPE) correctly and translocating RPE65 for visual pigment regeneration. USH1C mutation is also conjectured to produce truncated harmonin protein in abnormal splicing that causes retinal degeneration.[2]
Diagnosis
At present, Usher syndrome is incurable. Consequently, it is important to diagnose children early before they develop worsening visual problems.
Genetic Testing: Definitive diagnosis of Usher Syndrome type 1 involves identifying a homozygous proband mutation in the setting of positive symptoms and family history. The first gene to identify using single-gene sequencing is MYO7A. Only if a MYO7A mutation is absent or present in one gene should gene targeted deletion/duplication for MYO7A be done. Multigene panel is also an option for identifying mutations in the other genes mentioned in the genetics section. If single-gene or multigene testing fails to show mutations, a comprehensive genomic testing may be used to identify a mutation.[11]
Acadian patients should be tested for p.Val72Glu in USH1C and Ashkenazi Jewish patients should be tested for p.Arg245Ter in PCDH15 if suspicious for Usher syndrome.[11]
Audiology: Identify abnormal hearing loss.[11]
Electroretinography: Identify abnormal retina cell responses in eye.[11]
Ophthalmoscopic examination: Identify signs of retinitis pigmentosa.[11]
Visual Field Testing: Identify abnormal visual field defects.[11]
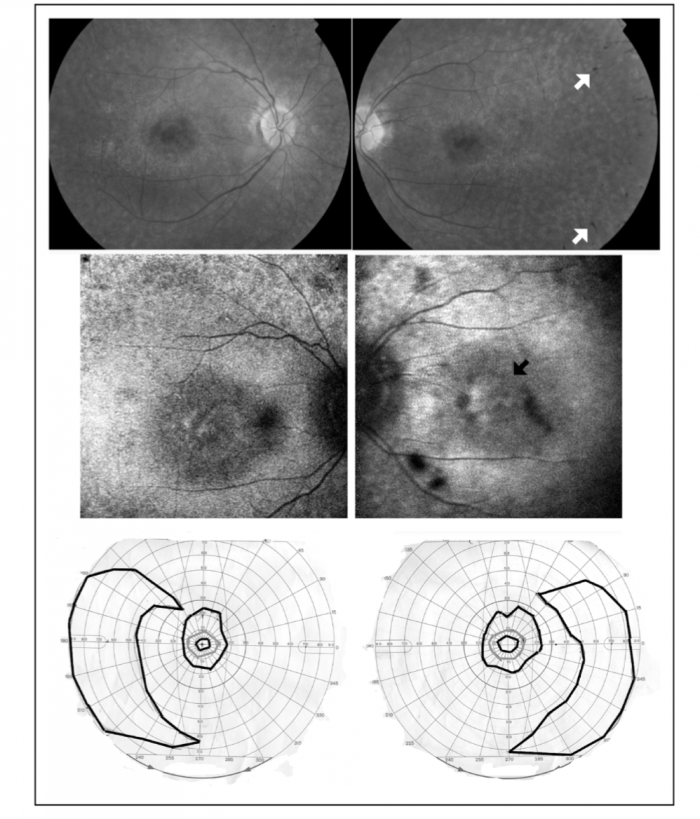
Clinical Presentation
Usher Syndrome I: Patients are born profoundly deaf and lose visual acuity within the first decade of life. Due to absent vestibular dysfunction, patients exhibit balance difficulty and as children learn to talk more slowly.[13]
Usher Syndrome II: In contrast to Type I, patient with Usher Syndrome II do not have congenital deafness. Rather, they have moderate-to-severe hearing loss that does not degrade over time. Furthermore, these patients do not exhibit any difficulty with balancing. Onset of visual loss in these patients is in the second decade of life. These patients may also have preserved visual acuity into the third and fourth decades of life.[2]
Usher Syndrome III: Similar to Type II, patients with Usher Syndrome III do not have congenital deafness. Instead they experience progressive hearing loss. Additionally, they exhibit varying degrees of balance issues due to mild to moderate vestibular dysfunction.[13]
Differential Diagnosis
- Nonsyndromic Hearing Loss
- Deafness-Dystonia-Optic Neuronopathy Syndrome
- Alport Syndrome
- Bardet--Biedl Syndrome
- Alstrom syndrome
- Cockayne Syndrome
- Friedreich Ataxia
- Hurler Syndrome
- Kearns-Sayre Syndrome
Management
Even though much progress has been made in elucidating the genetics and pathophysiology of Usher syndrome, there is still no cure. Treatment focuses on managing the auditory, balance, and visual manifestations.
Initial Diagnosis: The following evaluation modalities may be useful to establish the extent of the disease[11]:
- Audiology: Otoscopy, Pure tone audiometry, assessment of speech perception
- Vestibular Function: Rotary chair, calorics, electronystagmography, and computerized posturography
- Ophthalmology: Fundoscopy, visual acuity, visual field (Goldmann perimetry), and electroretinography
- Clinical Genetics: Clinical geneticist consultation
Treatment of Auditory Manifestations: Hearing aids provide limited benefit in patients with Usher I due to the severity of hearing loss, but can be helpful in patients with Usher II and III. Cochlear implants should be seriously considered in patients of all types.[14] Consider specialized training from educators of the hearing impaired.
Treatment of Vestibular Manifestations: Combination of RP which manifests as tunnel vision and night blindness with vestibular dysfunction predisposes patients to accidental injury. It is recommended that patients participate in well-supervised sports activities to compensate for balance problems by becoming more adept at somatosensation.[11]
Treatment of Visual Manifestations: Visual problems result from Retinitis Pigmentosa, which is currently incurable. However, recent advances in gene therapy have led to the development of numerous trials targeting genes of interest.[15] Currently, the only trial actively recruiting patients is for an intravitreal injection of an antisense oligonucleotide (ASO) to induce skipping of exon 13 in USH2A.[16] This requires patients to have a pathogenic exon 13 mutation in the USH2A gene to participate. Besides gene therapy, management involves the use of low vision aids, portable lighting, and potentially a guide dog if visual acuity loss is severe. Various supplements such as Vitamin A, DHA (docosahexaenoic acid), and Lutein may be helpful in delaying disease progression, but the literature is equivocal on efficacy.[17]
Surveillance: It is recommended that patients have routine ophthalmologic evaluation to detect possible complications of the disease process such as cataracts.
Genetic Counseling: Genetic evaluation is recommended for family members to determine genetic risk, carrier status, and to determine eligibility in clinical trials.
Prognosis
Usher I and II have congenital onset of hearing loss. As a result, hearing aids provide limited benefit. Because of the gradual progression of hearing loss in Usher Syndrome Type III hearing aids are often used successfully. Usher types I, II, and III have progressive loss of visual acuity by midlife.
Conclusion
Usher syndrome is a rare genetic condition that involves various degrees of hearing and vision loss over time. It is classified into three subtypes (I, II, and III) according to onset and severity of symptoms. Type II does not have vestibular dysfunction. It is at present incurable. However, gene therapy is one of the potential treatment modalities that are being researched and developed for potential future cures.[18]
Additional Resources
- Genetic and Rare Diseases Information Center (GARD). Usher syndrome. The Genetic and Rare Diseases (GARD) Information Center. https://rarediseases.info.nih.gov/diseases/7843/usher-syndrome/ Accessed 09 December 2019.
- Porter D, Vemulakonda GA. Usher Syndrome. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/usher-syndrome-list. Accessed December 09, 2019.
References
- ↑ Friedman TB, Schultz JM, Ahmed ZM, Tsilou ET, Brewer CC. Usher syndrome: hearing loss with vision loss. Adv Otorhinolaryngol. 2011;70:56-65.
- ↑ 2.0 2.1 2.2 2.3 Mathur P, Yang J. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852(3):406-20.
- ↑ Millán JM, Aller E, Jaijo T, Blanco-kelly F, Gimenez-pardo A, Ayuso C. An update on the genetics of usher syndrome. J Ophthalmol. 2011;2011:417217.
- ↑ Kimberling WJ, Hildebrand MS, Shearer AE, et al. Frequency of Usher syndrome in two pediatric populations: Implications for genetic screening of deaf and hard of hearing children. Genet Med. 2010;12(8):512-6.
- ↑ Vernon M. Usher's syndrome--deafness and progressive blindness. Clinical cases, prevention, theory and literature survey. J Chronic Dis. 1969;22(3):133-51.
- ↑ Boughman JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36(8):595-603.
- ↑ Ben-yosef T, Ness SL, Madeo AC, et al. A mutation of PCDH15 among Ashkenazi Jews with the type 1 Usher syndrome. N Engl J Med. 2003;348(17):1664-70.
- ↑ Ebermann I, Koenekoop RK, Lopez I, Bou-khzam L, Pigeon R, Bolz HJ. An USH2A founder mutation is the major cause of Usher syndrome type 2 in Canadians of French origin and confirms common roots of Quebecois and Acadians. Eur J Hum Genet. 2009;17(1):80-4.
- ↑ NIH. Usher Syndrome. Genetics Home Reference. July 9, 2019.
- ↑ Delmaghani S, El-Amraoui A. The genetic and phenotypic landscapes of Usher syndrome: from disease mechanisms to a new classification. Hum Genet. 2022 Apr;141(3-4):709-735. doi: 10.1007/s00439-022-02448-7. Epub 2022 Mar 30. PMID: 35353227; PMCID: PMC9034986.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 Lentz J, Keats B, Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K. Usher Syndrome Type I, Usher Syndrome Type II. GeneReviews®. 1999 Dec 10 [updated 2016 Jul 21].
- ↑ Saihan Z, Webster AR, Luxon L, Bitner-glindzicz M. Update on Usher syndrome. Curr Opin Neurol. 2009;22(1):19-27.
- ↑ 13.0 13.1 Mets MB, Young NM, Pass A, Lasky JB. Early diagnosis of Usher syndrome in children. Trans Am Ophthalmol Soc. 2000;98:237-42.
- ↑ Damon, G., Pennings, R., Snik, A., & Mylanus, E. (2006). Quality of life and cochlear implantation in Usher syndrome type I. Laryngoscope, 116, 723–728
- ↑ Toms M, Pagarkar W, Moosajee M. Usher syndrome: clinical features, molecular genetics and advancing therapeutics. Ther Adv Ophthalmol. 2020 Sep 17;12:2515841420952194. doi: 10.1177/2515841420952194. PMID: 32995707; PMCID: PMC7502997.
- ↑ ProQR Therapeutics. Study to Evaluate the Efficacy Safety and Tolerability of QR-421a in Subjects With RP Due to Mutations in Exon 13 of the USH2A Gene With Advanced Vision Loss (Sirius). ClinicalTrials.gov identifier: NCT05158296. Updated July 14, 2022. Accessed July 19, 2022. clinicaltrials.gov
- ↑ Berson EL, Rosner B, Sandberg MA, et al: A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993; 111(6):761-772.
- ↑ Aparisi MJ, Aller E, Fuster-garcía C, et al. Targeted next generation sequencing for molecular diagnosis of Usher syndrome. Orphanet J Rare Dis. 2014;9:168.

