Optic Nerve Coloboma Spectrum: Difference between revisions
| Line 9: | Line 9: | ||
= Overview = | = Overview = | ||
Optic Nerve Coloboma Spectrum comprises a spectrum of diseases with overlapping characteristics (cavitation of the optic disc), but overall different clinical findings, etiologies, and associations. Some of the diseases discussed are Optic Disc coloboma, Morning Glory Syndrome, Peripapillary Staphyloma, Pedler Coloboma, and Optic Disc pits. | Optic Nerve Coloboma Spectrum comprises a spectrum of diseases with overlapping characteristics (cavitation of the optic disc), but overall different clinical findings, etiologies, and associations. Some of the diseases discussed are Optic Disc coloboma, Morning Glory Syndrome, Peripapillary Staphyloma, Pedler Coloboma, and Optic Disc pits.<ref name="dutton1">Dutton G. Congenital disorders of the optic nerve: excavations and hypoplasia. Eye. 2004;18:1038–1048</ref> | ||
== Nomenclature == | == Nomenclature == | ||
The nomenclature regarding the spectrum of diseases can be confusing and include “Optic Disc Cavitary Lesions,” “Excavated Optic Disk anomalies,” and “Optic Nerve Coloboma Spectrum.” In the literature and in this EyeWiki we use the terms “Optic Disc” coloboma and “Optic Nerve” coloboma interchangeably. | The nomenclature regarding the spectrum of diseases can be confusing and include “Optic Disc Cavitary Lesions,” “Excavated Optic Disk anomalies,” and “Optic Nerve Coloboma Spectrum.” In the literature and in this EyeWiki we use the terms “Optic Disc” coloboma and “Optic Nerve” coloboma interchangeably.<ref name="dutton1"/> | ||
== Management == | == Management == | ||
Management of optic disc excavations is focused on early detection and visual correction for amblyopia (e.g., the trial of patching or early optimal refraction in optic disc coloboma) and awareness of common associations/complications for proactive management (e.g., neuroimaging in morning glory syndrome for associated basoencepholcele). There is no treatment or prevention for cavitary disc anomalies. | Management of optic disc excavations is focused on early detection and visual correction for amblyopia (e.g., the trial of patching or early optimal refraction in optic disc coloboma) and awareness of common associations/complications for proactive management (e.g., neuroimaging in morning glory syndrome for associated basoencepholcele). There is no treatment or prevention for cavitary disc anomalies.<ref name="dutton1"/><ref name="lee2">Lee BJ, Elias IT. Update on the Morning Glory Disc Anomaly. Ophthalmic genetics 2008;29.2: 47–52</ref> | ||
| Line 25: | Line 25: | ||
== Clinical findings/Physical Examination == | == Clinical findings/Physical Examination == | ||
The optic disc coloboma (ODC) is characterized by a bowl shaped excavation often inferiorly with sharp borders. Unlike the morning glory disc, the ODC has no central glial tuft and the disc vasculature is usually normal. ODC can mimic glaucomatous cupping | The optic disc coloboma (ODC) is characterized by a bowl shaped excavation often inferiorly with sharp borders. Unlike the morning glory disc, the ODC has no central glial tuft and the disc vasculature is usually normal. ODC can mimic glaucomatous cupping<ref name="dutton1"/><ref name="lingam3">Lingam G, Badrinath SS, Kumar KS, Doshi G, Biswas N. Optic disc in fundus coloboma. Ophthalmology. 1996;103:2120–2126</ref> but the excavation in ODC is normally decentered inferiorly and is non-progressive.<ref name="brodsky4">Brodsky MC. Congenital Optic Disk Anomalies. Survey of Ophthalmology. 1994;39.2:89–112</ref> Microphthalmia can be present if the ODC related excavation includes the retina or choroid. Visual acuity in ODC can be difficult to predict from the appearance of the disc alone and can range from mild to severely affected vision. Unilateral or bilateral ODC may occur.<ref name="pollock5">Pollock S. The morning glory disc anomaly: Contractile movement, classification, and embryogenesis. Documenta Ophthalmologica. 1987;65:439-460</ref> | ||
== Etiology == | == Etiology == | ||
ODC is believed to be the result of incomplete closure of the proximal part of the embryonal fissure during the sixth week of gestation. | ODC is believed to be the result of incomplete closure of the proximal part of the embryonal fissure during the sixth week of gestation.<ref name="duvall6">Duvall J, Miller SL, Cheatle E, Tso MO. Histopathologic study of ocular changes in a syndrome of multiple congenital anomalies. Am J Ophthalmol. 1987;103:701–705</ref> PAX2 genes, expressed in astrocytic cells, are also expressed in adult retinal cells located on the optic nerve head. Precursor astrocytes are found on the edge of the optic nerve during early development – forming the theory that abnormal astrocyte differentiation or migration during development may play a role in optic nerve coloboma formation.<ref name="chu7">Chu Y, Hughes S, Chan-Ling T. Differentiation and migration of astrocyte precursor cells and astrocytes in human fetal retina: relevance to optic nerve coloboma. FASEB J. 2001;15:2013–2015</ref> | ||
== Ancillary testing == | == Ancillary testing == | ||
One study of ODC used swept-source optical coherence tomography (OCT) and described sparse and irregularly oriented scleral fibers and openings of the subarachnoid space just posterior to the bottom of the excavation. | One study of ODC used swept-source optical coherence tomography (OCT) and described sparse and irregularly oriented scleral fibers and openings of the subarachnoid space just posterior to the bottom of the excavation.<ref name="ohnomatsui8">Ohno-Matsui K, Hirakata A, Inoue M, Akiba M, Ishibashi T. Evaluation of Congenital Optic Disc Pits and Optic Disc Colobomas by Swept-Source Optical Coherence Tomography. Investigative ophthalmology & visual science 2013;54(12): 7769–7778</ref> | ||
[[File:AA0 nerve coloboma 2019.jpg|alt=Optic nerve coloboma. Available at AAO.org. Last accessed Sep 5, 2022.|left|thumb|Optic nerve coloboma. Available at AAO.org. Last accessed Sep 5, 2022.]] | [[File:AA0 nerve coloboma 2019.jpg|alt=Optic nerve coloboma. Available at AAO.org. Last accessed Sep 5, 2022.|left|thumb|Optic nerve coloboma. Available at AAO.org. Last accessed Sep 5, 2022.]] | ||
== Associations == | == Associations == | ||
ODC can be associated with a number of genetic disorders (e.g., PAX gene mutations, Walker-Walburg syndrome, Aicardi syndrome). Isolated ODC without systemic abnormalities can be sporadic or familial | ODC can be associated with a number of genetic disorders (e.g., PAX gene mutations, Walker-Walburg syndrome, Aicardi syndrome). Isolated ODC without systemic abnormalities can be sporadic or familial<ref name="lingam9">Lingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. Eye (Lond). 2021;35(8):2086-2109</ref> There is no known racial or sexual predilection in ODC.<ref name="lingam3"/> An important association is papillorenal syndrome. Few isolated cases with both anterior and posterior coloboma have been described in Tuberous sclerosis<ref>Bacci, Giacomo M. MD, PhD; Polizzi, Silvio MD; Mari, Francesco MD, PhD; Conti, Valerio PhD; Caputo, Roberto MD; Guerrini, Renzo MD, FRCP. Atypical Ocular Coloboma in Tuberous Sclerosis-2: Report of Two Novel Cases. Journal of Neuro-Ophthalmology 41(3):p e363-e365, September 2021. | DOI: 10.1097/WNO.0000000000001099 </ref>. | ||
=[[Papillorenal_Syndrome|Papillorenal syndrome]] (Renal-coloboma syndrome)= | =[[Papillorenal_Syndrome|Papillorenal syndrome]] (Renal-coloboma syndrome)= | ||
==Clinical Findings/PE== | ==Clinical Findings/PE== | ||
Optic disc excavations in the papillorenal syndrome commonly lack central retinal vessels. | Optic disc excavations in the papillorenal syndrome commonly lack central retinal vessels.<ref name="khan10">Khan AO, Nowilaty SR. Early diagnosis of the papillorenal syndrome by optic disc morphology. J Neuroophthalmol. 2005;25(3):209-211</ref><ref name="ng11">Ng B, De Silva S, Bindra M. Papillorenal syndrome: a systemic diagnosis not to be missed on funduscopy. BMJ Case Rep. 2021;14(7):e241708</ref> <ref name="parsa12">Parsa CF, Silva ED, Sundin OH, et al. Redefining papillorenal syndrome: an underdiagnosed cause of ocular and renal morbidity. Ophthalmology. 2001;108(4):738-749</ref> There are usually multiple cilioretinal vessels surrounding the optic disc and arising from its periphery, typically inferotermporally.<ref name="khan10"/> These can lead to static nonarcuate superonasal field defects with steep contracted isopters with geographic borders.<ref name="parsa12"/> Ocular findings of papillorenal syndrome are commonly mistaken for glaucoma, colobomas, or morning glory anomalies – awareness of the vasculature can help differentiate it from these.<ref name="parsa12"/> Visual acuity is normally unaffected. | ||
==Etiology== | ==Etiology== | ||
This syndrome’s alternative name, “renal-coloboma syndrome,” is somewhat of a misnomer as there is no evidence that failure in the closure of the optic fissure is related to its etiology like it is with optic disc coloboma. | This syndrome’s alternative name, “renal-coloboma syndrome,” is somewhat of a misnomer as there is no evidence that failure in the closure of the optic fissure is related to its etiology like it is with optic disc coloboma.<ref name="parsa12"/> The common anomaly found in papillorenal syndrome is optic disc dysplasia.<ref name="khan10"/> <ref name="ng11"/> <ref name="parsa12"/> This finding is thought to be secondary to abnormal angiogenesis, leading to the compromised growth of the retina and choroid.<ref name="parsa12"/> | ||
Similarly to optic disc colobomas, papillorenal syndrome is related to mutations of the PAX2 gene. | Similarly to optic disc colobomas, papillorenal syndrome is related to mutations of the PAX2 gene.<ref name="khan10"/> <ref name="ng11"/> <ref name="parsa12"/> It’s hypothesized that some PAX2 mutations may lead to abnormal development of the optic stalk resulting in abnormal vascularity on the optic nerve head.<ref name="parsa12"/> One study analyzing PAX2 mutations in 11 families with the syndrome failed to detect PAX2 mutations in 3 of the families; suggesting that genetic heterogeneity (mutations in several other genes responsible for the phenotype) is responsible for some cases.<ref name="parsa12"/> | ||
==Associations== | ==Associations== | ||
Peripheral disc excavations that disrupt glial border tissue can lead to cerebrospinal fluid flow and subsequent serous retinal detachment. | Peripheral disc excavations that disrupt glial border tissue can lead to cerebrospinal fluid flow and subsequent serous retinal detachment.<ref name="khan10"/> PAX2 disruption also leads to abnormal mesenchymal differentiation of renal and urinary tracts – this hypodysplasia can lead to renal hypoplasia, hypertension, and failure.<ref name="parsa12"/> Additionally, patients with papillorenal syndrome have been found to have bilateral neurosensory hearing loss, laxity of the ligaments, and sometimes neurological malformations and epilepsy.<ref name="khan10"/> | ||
| Line 53: | Line 53: | ||
==Clinical Findings/PE == | ==Clinical Findings/PE == | ||
The morning glory disc (MGD) is so named because it resembles the morning glory flower. The key ophthalmoscopic features of the MGD are a funnel-shaped excavation with a tuft of glial tissue lying at the center of the excavation and a dramatic and increased retinal vascularity radiating outwards from the periphery of the optic disc. | The morning glory disc (MGD) is so named because it resembles the morning glory flower. The key ophthalmoscopic features of the MGD are a funnel-shaped excavation with a tuft of glial tissue lying at the center of the excavation and a dramatic and increased retinal vascularity radiating outwards from the periphery of the optic disc.<ref name="lee2"/> MGD is typically unilateral, and the visual acuity is poor (typically ranging from 20/200 to finger counting). | ||
==Etiology== | ==Etiology== | ||
The exact etiology of the MGD is unclear. Still, the most accepted hypothesis suggests that the pathogenesis may be similar to that of ODC with a defective closure of the embryonic fissure. Some authors believe that MGD and ODC are on the same phenotypic spectrum of colobomatous disc defects. | The exact etiology of the MGD is unclear. Still, the most accepted hypothesis suggests that the pathogenesis may be similar to that of ODC with a defective closure of the embryonic fissure. Some authors believe that MGD and ODC are on the same phenotypic spectrum of colobomatous disc defects.<ref name="gardner13">Gardner TW, Zaparackas ZG, Naidich TP. Congenital optic nerve colobomas: CT demonstration. J Comput Assist Tomogr. 1984;8:95-102</ref><ref name="pollock5"/> | ||
[[File:Image 2.png|thumb|304x304px|Photographs of Morning Glory Disc - The white arrow points at the tuft of glial tissue at the center. The orange arrow at the bottom of the image points to the relatively straight blood vessels from the optic disc. The left inset photo shows retinal detachment in an eye with morning glory syndrome and the right inset photo is an ultrasound showing the funnel-shaped posterior excavation. Photo credit: Lingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. ''Eye (Lond)''. 2021;35(8):2086-2109. doi:10.1038/s41433-021-01501-5; This work is licensed under a Creative Commons Attribution 4.0 International License. No changes were made to this image.]] | [[File:Image 2.png|thumb|304x304px|Photographs of Morning Glory Disc - The white arrow points at the tuft of glial tissue at the center. The orange arrow at the bottom of the image points to the relatively straight blood vessels from the optic disc. The left inset photo shows retinal detachment in an eye with morning glory syndrome and the right inset photo is an ultrasound showing the funnel-shaped posterior excavation. Photo credit: Lingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. ''Eye (Lond)''. 2021;35(8):2086-2109. doi:10.1038/s41433-021-01501-5; This work is licensed under a Creative Commons Attribution 4.0 International License. No changes were made to this image.]] | ||
==Associations== | ==Associations== | ||
The classic association of MGD is the basal encephalocele (an outpouching of the meninges). Additional associations with MGD are cerebrovascular anomalies, PHACE (Posterior fossa, Hemangioma, Arterial lesions, Cardiac abnormalities, Eye abnormalities) syndrome, and neurofibromatosis type 2. | The classic association of MGD is the basal encephalocele (an outpouching of the meninges). Additional associations with MGD are cerebrovascular anomalies, PHACE (Posterior fossa, Hemangioma, Arterial lesions, Cardiac abnormalities, Eye abnormalities) syndrome, and neurofibromatosis type 2.<ref name="brodsky4"/> In contrast to ODC, MGD is more common in females and Caucasians but has rarely been reported in African Americans.<ref name="lingam3"/> | ||
| Line 66: | Line 66: | ||
==Clinical Findings/PE == | ==Clinical Findings/PE == | ||
The optic disc usually looks normal in cases of peripapillary staphyloma (PPS). PPS may be unilateral or bilateral with atrophic pigmentary changes. | The optic disc usually looks normal in cases of peripapillary staphyloma (PPS). PPS may be unilateral or bilateral with atrophic pigmentary changes.<ref name="wise14">Wise JB, Maclean AL, Gass JD. Contractile peripapillary staphyloma. Arch Ophthalmol 1966;75:626-630</ref> The vascularity of the disc in PPS is normal and differs from the ODC and MGD.<ref name="lee2"/> Visual acuity can range from markedly reduced to normal depending on the involvement of the macular and those with decreased vision frequently have centrocecal scotomas.<ref name="wise14"/> Transient vision loss may occur, a recent study suggests that contractions, secondary to either pressure imbalance or muscle contractions, leading to a decrease in vessel density which may be responsible for the transient vision loss. Spontaneous contractile movements of the optic disc region have been seen also in ODC and MGD.<ref name="brodsky15">Brodsky MC. Contractile morning glory disc causing transient monocular blindness in a child. Arch Ophthalmology. 2006;8:1199-1201</ref> <ref name="graether16">Graether JM. Transient amaurosis in one eye with simultaneous dilatation of retinal veins in association with a congenital anomaly of the optic nerve head. Arch Ophthalmology. 1963;70:342-345</ref> <ref name="hokazono17">Hokazono K, Carstens LD, Monteiro RB. Contractile Peripapillary Staphyloma: OCTA Documentation of Increased Peripapillary Vessel Density During Transient Visual Loss Episodes. American journal of ophthalmology case reports. 2021;21</ref> | ||
==Ancillary testing== | ==Ancillary testing== | ||
The PPS demonstrates on ultrasound, OCT, and fundus exam peripapillary atrophy and excavation with thinning o of the retinal layers around the posterior staphyloma and steep slope to the bottom of the disc. | The PPS demonstrates on ultrasound, OCT, and fundus exam peripapillary atrophy and excavation with thinning o of the retinal layers around the posterior staphyloma and steep slope to the bottom of the disc.<ref name="hokazono17"/> | ||
== Etiology == | == Etiology == | ||
The suggested etiology is that it is due to diminished peripapillary structural support resulting from incomplete differentiation of sclera from posterior neural crest cells at 5 months gestation. Once there is the establishment of normal intraocular pressure the PPS is formed via herniation of unsupported ocular tissues through the defect. | The suggested etiology is that it is due to diminished peripapillary structural support resulting from incomplete differentiation of sclera from posterior neural crest cells at 5 months gestation. Once there is the establishment of normal intraocular pressure the PPS is formed via herniation of unsupported ocular tissues through the defect.<ref name="brodsky4"/><ref name="cogan18">Cogan DG. Coloboma of Optic Nerve with Overlay of Peripapillary Retina. Brittish journal of ophthalmology 1978;62(6):347–350</ref> | ||
== Associations/complications == | == Associations/complications == | ||
[[File:Image 3.png|thumb|530x530px|Fundus photographs demonstrating peripapillary staphyloma – A: Normal disc surrounded by peripapillary staphyloma. B: Close inspection reveals the optic disc is normal, as outlined by the arrows. The inset photograph shows a typical optic disc coloboma for comparison. Photo credit: Lingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. ''Eye (Lond)''. 2021;35(8):2086-2109. doi:10.1038/s41433-021-01501-5; This work is licensed under a Creative Commons Attribution 4.0 International License. No changes were made to this image.]] | [[File:Image 3.png|thumb|530x530px|Fundus photographs demonstrating peripapillary staphyloma – A: Normal disc surrounded by peripapillary staphyloma. B: Close inspection reveals the optic disc is normal, as outlined by the arrows. The inset photograph shows a typical optic disc coloboma for comparison. Photo credit: Lingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. ''Eye (Lond)''. 2021;35(8):2086-2109. doi:10.1038/s41433-021-01501-5; This work is licensed under a Creative Commons Attribution 4.0 International License. No changes were made to this image.]] | ||
Some case reports have described retinal detachment around the optic disc in eyes with PPS. | Some case reports have described retinal detachment around the optic disc in eyes with PPS.<ref name="cao19">Cao XS, Peng XY, You QS, et al. Congenital contractile peripapillary staphyloma with rhegmatogenous retinal detachment. Retin Cases Brief Rep. 2016;12(1):48–49</ref> <ref name="kunita20">Kunita D, Inoue M, Koto T, Hirakata A. Retinal detachment associated with peripapillary staphyloma imaged with swept source optical coherence tomography. Retin Cases Brief Rep. 2017;13(1):25–29</ref> | ||
| Line 82: | Line 82: | ||
==Clinical Findings/PE== | ==Clinical Findings/PE== | ||
Described first by Pedler in 1961, Pedler Coloboma (PC) has been described as an optic disc with heterotopic adipose tissue and smooth muscle). The PC presents as an abnormally wide scleral canal filled with connective tissue. | Described first by Pedler in 1961, Pedler Coloboma (PC) has been described as an optic disc with heterotopic adipose tissue and smooth muscle). The PC presents as an abnormally wide scleral canal filled with connective tissue.<ref name="cogan18"/><ref name="willis21">Willis R, Zimmerman LE, O'Grady R, Smith RS, Crawford B. Heterotopic Adipose Tissue and Smooth Muscle in the Optic Disc: Association With Isolated Colobomas. Arch Ophthalmol. 1972;88(2):139–146.</ref> Buckling of the retina can form an epipapillary mass that may be mistaken for an ocular tumor. The PC is described as loose richly vascularized connective tissue located in the region of the physiological optic cup.<ref name="cogan18"/> The optic disc can be seen synchronously pulsating with respiration.<ref name="cogan18"/><ref name="warburg22">Warburg M. Classification of microphthalmos and coloboma. Journal of Medical Genetics 1993;30:664-669</ref> | ||
==Etiology== | ==Etiology== | ||
PC is believed to result from a failure of maturation in the posterior sclera (secondary to derangement of the optic cup) leading to failed fusion of the peripapillary retina, optic disc, lamina cribrosa, and posterior choroid. These resultant layers are then driven posteriorly through the resultant hiatus. | PC is believed to result from a failure of maturation in the posterior sclera (secondary to derangement of the optic cup) leading to failed fusion of the peripapillary retina, optic disc, lamina cribrosa, and posterior choroid. These resultant layers are then driven posteriorly through the resultant hiatus.<ref name="cogan18"/> | ||
==Associations== | ==Associations== | ||
Cases including these heterotopic mesodermal characteristics described by Willis et al. were all in association with isolated colobomas. | Cases including these heterotopic mesodermal characteristics described by Willis et al. were all in association with isolated colobomas.<ref name="willis21"/> | ||
== Histopathological analysis== | == Histopathological analysis== | ||
Commonly a large scleral canal will be seen with absence or defective formation of the lamina cribosa. Glial tissue and hyperplastic pigment epithelium is present between the optic nerve and the sclera. | Commonly a large scleral canal will be seen with absence or defective formation of the lamina cribosa. Glial tissue and hyperplastic pigment epithelium is present between the optic nerve and the sclera.<ref name="chu7"/> | ||
=[[Optic Pits|Optic pit]] (OP)= | =[[Optic Pits|Optic pit]] (OP)= | ||
==Clinical Findings/PE== | ==Clinical Findings/PE== | ||
The optic pit (OP) is a crater-like depression of the optic nerve head. | The optic pit (OP) is a crater-like depression of the optic nerve head.<ref name="duvall6"/> OP has no systemic findings and is commonly found on the temporal side of the disc.<ref name="duvall6"/> Depending on the size of the OP and its location there may or may not be visual field loss (e.g., arcuate field defects) but visual acuity is typically not affected.<ref name="gordon23">Gordon R, Chatfield RK. Pits in the optic disc associated with macular degeneration. The British journal of ophthalmology. 1969;53(7):481–9.</ref> | ||
==Etiology == | ==Etiology == | ||
The etiology of the OP is thought to be similar to ODC and MGD with incomplete closure of the superior edge of the embryonic fissure. | The etiology of the OP is thought to be similar to ODC and MGD with incomplete closure of the superior edge of the embryonic fissure.<ref name="reed24">Reed D. Congenital pits of the optic nerve. Clin Eye Vis Care. 1999;11(2):75–80</ref> | ||
== Complications/Associations== | == Complications/Associations== | ||
Common complications of the OP are serous retinal detachment and/or retinoschisis in the area of the macular, this is called OP maculopathy. | Common complications of the OP are serous retinal detachment and/or retinoschisis in the area of the macular, this is called OP maculopathy.<ref name="reed24"/> | ||
==Management and treatment== | ==Management and treatment== | ||
If the macula is affected with serous detachment in OP then treatment modalities include laser photocoagulation to create a barrier between the disc and the macula so fluid doesn’t enter the macula, pars plana vitrectomy (greater success than photocoagulation), injecting gas tamponade, and/or macular buckle. | If the macula is affected with serous detachment in OP then treatment modalities include laser photocoagulation to create a barrier between the disc and the macula so fluid doesn’t enter the macula, pars plana vitrectomy (greater success than photocoagulation), injecting gas tamponade, and/or macular buckle.<ref name="chatziralli25">Chatziralli I, Theodossiadis P, Theodossiadis GP. Optic disk pit maculopathy: Current management strategies. Clinical Ophthalmology. 2018;12:1417–1422</ref> | ||
=Summary= | =Summary= | ||
Clinicians should recognize the spectrum of optic disc related cavitary disc anomalies and neuroimaging may be necessary of some cases to exclude associated intracranial defects (e.g., basal encephalocoele). | Clinicians should recognize the spectrum of optic disc related cavitary disc anomalies and neuroimaging may be necessary of some cases to exclude associated intracranial defects (e.g., basal encephalocoele). | ||
=References= | =References= | ||
<references /> | <references /> | ||
Revision as of 14:08, July 19, 2023
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Overview
Optic Nerve Coloboma Spectrum comprises a spectrum of diseases with overlapping characteristics (cavitation of the optic disc), but overall different clinical findings, etiologies, and associations. Some of the diseases discussed are Optic Disc coloboma, Morning Glory Syndrome, Peripapillary Staphyloma, Pedler Coloboma, and Optic Disc pits.[1]
Nomenclature
The nomenclature regarding the spectrum of diseases can be confusing and include “Optic Disc Cavitary Lesions,” “Excavated Optic Disk anomalies,” and “Optic Nerve Coloboma Spectrum.” In the literature and in this EyeWiki we use the terms “Optic Disc” coloboma and “Optic Nerve” coloboma interchangeably.[1]
Management
Management of optic disc excavations is focused on early detection and visual correction for amblyopia (e.g., the trial of patching or early optimal refraction in optic disc coloboma) and awareness of common associations/complications for proactive management (e.g., neuroimaging in morning glory syndrome for associated basoencepholcele). There is no treatment or prevention for cavitary disc anomalies.[1][2]
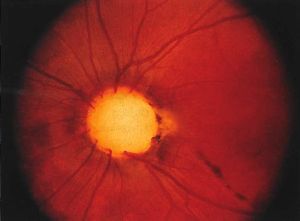
Optic Disc coloboma
Clinical findings/Physical Examination
The optic disc coloboma (ODC) is characterized by a bowl shaped excavation often inferiorly with sharp borders. Unlike the morning glory disc, the ODC has no central glial tuft and the disc vasculature is usually normal. ODC can mimic glaucomatous cupping[1][3] but the excavation in ODC is normally decentered inferiorly and is non-progressive.[4] Microphthalmia can be present if the ODC related excavation includes the retina or choroid. Visual acuity in ODC can be difficult to predict from the appearance of the disc alone and can range from mild to severely affected vision. Unilateral or bilateral ODC may occur.[5]
Etiology
ODC is believed to be the result of incomplete closure of the proximal part of the embryonal fissure during the sixth week of gestation.[6] PAX2 genes, expressed in astrocytic cells, are also expressed in adult retinal cells located on the optic nerve head. Precursor astrocytes are found on the edge of the optic nerve during early development – forming the theory that abnormal astrocyte differentiation or migration during development may play a role in optic nerve coloboma formation.[7]
Ancillary testing
One study of ODC used swept-source optical coherence tomography (OCT) and described sparse and irregularly oriented scleral fibers and openings of the subarachnoid space just posterior to the bottom of the excavation.[8]
Associations
ODC can be associated with a number of genetic disorders (e.g., PAX gene mutations, Walker-Walburg syndrome, Aicardi syndrome). Isolated ODC without systemic abnormalities can be sporadic or familial[9] There is no known racial or sexual predilection in ODC.[3] An important association is papillorenal syndrome. Few isolated cases with both anterior and posterior coloboma have been described in Tuberous sclerosis[10].
Papillorenal syndrome (Renal-coloboma syndrome)
Clinical Findings/PE
Optic disc excavations in the papillorenal syndrome commonly lack central retinal vessels.[11][12] [13] There are usually multiple cilioretinal vessels surrounding the optic disc and arising from its periphery, typically inferotermporally.[11] These can lead to static nonarcuate superonasal field defects with steep contracted isopters with geographic borders.[13] Ocular findings of papillorenal syndrome are commonly mistaken for glaucoma, colobomas, or morning glory anomalies – awareness of the vasculature can help differentiate it from these.[13] Visual acuity is normally unaffected.
Etiology
This syndrome’s alternative name, “renal-coloboma syndrome,” is somewhat of a misnomer as there is no evidence that failure in the closure of the optic fissure is related to its etiology like it is with optic disc coloboma.[13] The common anomaly found in papillorenal syndrome is optic disc dysplasia.[11] [12] [13] This finding is thought to be secondary to abnormal angiogenesis, leading to the compromised growth of the retina and choroid.[13] Similarly to optic disc colobomas, papillorenal syndrome is related to mutations of the PAX2 gene.[11] [12] [13] It’s hypothesized that some PAX2 mutations may lead to abnormal development of the optic stalk resulting in abnormal vascularity on the optic nerve head.[13] One study analyzing PAX2 mutations in 11 families with the syndrome failed to detect PAX2 mutations in 3 of the families; suggesting that genetic heterogeneity (mutations in several other genes responsible for the phenotype) is responsible for some cases.[13]
Associations
Peripheral disc excavations that disrupt glial border tissue can lead to cerebrospinal fluid flow and subsequent serous retinal detachment.[11] PAX2 disruption also leads to abnormal mesenchymal differentiation of renal and urinary tracts – this hypodysplasia can lead to renal hypoplasia, hypertension, and failure.[13] Additionally, patients with papillorenal syndrome have been found to have bilateral neurosensory hearing loss, laxity of the ligaments, and sometimes neurological malformations and epilepsy.[11]
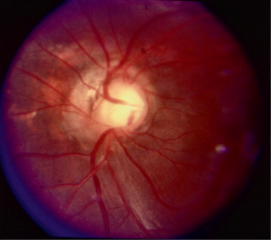
Morning Glory Disc (MGD)
Clinical Findings/PE
The morning glory disc (MGD) is so named because it resembles the morning glory flower. The key ophthalmoscopic features of the MGD are a funnel-shaped excavation with a tuft of glial tissue lying at the center of the excavation and a dramatic and increased retinal vascularity radiating outwards from the periphery of the optic disc.[2] MGD is typically unilateral, and the visual acuity is poor (typically ranging from 20/200 to finger counting).
Etiology
The exact etiology of the MGD is unclear. Still, the most accepted hypothesis suggests that the pathogenesis may be similar to that of ODC with a defective closure of the embryonic fissure. Some authors believe that MGD and ODC are on the same phenotypic spectrum of colobomatous disc defects.[14][5]
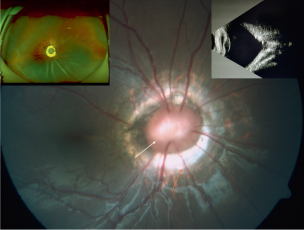
Associations
The classic association of MGD is the basal encephalocele (an outpouching of the meninges). Additional associations with MGD are cerebrovascular anomalies, PHACE (Posterior fossa, Hemangioma, Arterial lesions, Cardiac abnormalities, Eye abnormalities) syndrome, and neurofibromatosis type 2.[4] In contrast to ODC, MGD is more common in females and Caucasians but has rarely been reported in African Americans.[3]
Peripapillary Staphyloma
Clinical Findings/PE
The optic disc usually looks normal in cases of peripapillary staphyloma (PPS). PPS may be unilateral or bilateral with atrophic pigmentary changes.[15] The vascularity of the disc in PPS is normal and differs from the ODC and MGD.[2] Visual acuity can range from markedly reduced to normal depending on the involvement of the macular and those with decreased vision frequently have centrocecal scotomas.[15] Transient vision loss may occur, a recent study suggests that contractions, secondary to either pressure imbalance or muscle contractions, leading to a decrease in vessel density which may be responsible for the transient vision loss. Spontaneous contractile movements of the optic disc region have been seen also in ODC and MGD.[16] [17] [18]
Ancillary testing
The PPS demonstrates on ultrasound, OCT, and fundus exam peripapillary atrophy and excavation with thinning o of the retinal layers around the posterior staphyloma and steep slope to the bottom of the disc.[18]
Etiology
The suggested etiology is that it is due to diminished peripapillary structural support resulting from incomplete differentiation of sclera from posterior neural crest cells at 5 months gestation. Once there is the establishment of normal intraocular pressure the PPS is formed via herniation of unsupported ocular tissues through the defect.[4][19]
Associations/complications
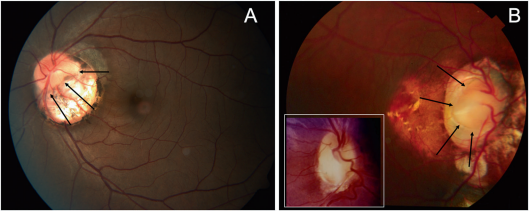
Some case reports have described retinal detachment around the optic disc in eyes with PPS.[20] [21]
Pedler coloboma
Clinical Findings/PE
Described first by Pedler in 1961, Pedler Coloboma (PC) has been described as an optic disc with heterotopic adipose tissue and smooth muscle). The PC presents as an abnormally wide scleral canal filled with connective tissue.[19][22] Buckling of the retina can form an epipapillary mass that may be mistaken for an ocular tumor. The PC is described as loose richly vascularized connective tissue located in the region of the physiological optic cup.[19] The optic disc can be seen synchronously pulsating with respiration.[19][23]
Etiology
PC is believed to result from a failure of maturation in the posterior sclera (secondary to derangement of the optic cup) leading to failed fusion of the peripapillary retina, optic disc, lamina cribrosa, and posterior choroid. These resultant layers are then driven posteriorly through the resultant hiatus.[19]
Associations
Cases including these heterotopic mesodermal characteristics described by Willis et al. were all in association with isolated colobomas.[22]
Histopathological analysis
Commonly a large scleral canal will be seen with absence or defective formation of the lamina cribosa. Glial tissue and hyperplastic pigment epithelium is present between the optic nerve and the sclera.[7]
Optic pit (OP)
Clinical Findings/PE
The optic pit (OP) is a crater-like depression of the optic nerve head.[6] OP has no systemic findings and is commonly found on the temporal side of the disc.[6] Depending on the size of the OP and its location there may or may not be visual field loss (e.g., arcuate field defects) but visual acuity is typically not affected.[24]
Etiology
The etiology of the OP is thought to be similar to ODC and MGD with incomplete closure of the superior edge of the embryonic fissure.[25]
Complications/Associations
Common complications of the OP are serous retinal detachment and/or retinoschisis in the area of the macular, this is called OP maculopathy.[25]
Management and treatment
If the macula is affected with serous detachment in OP then treatment modalities include laser photocoagulation to create a barrier between the disc and the macula so fluid doesn’t enter the macula, pars plana vitrectomy (greater success than photocoagulation), injecting gas tamponade, and/or macular buckle.[26]
Summary
Clinicians should recognize the spectrum of optic disc related cavitary disc anomalies and neuroimaging may be necessary of some cases to exclude associated intracranial defects (e.g., basal encephalocoele).
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Dutton G. Congenital disorders of the optic nerve: excavations and hypoplasia. Eye. 2004;18:1038–1048
- ↑ Jump up to: 2.0 2.1 2.2 Lee BJ, Elias IT. Update on the Morning Glory Disc Anomaly. Ophthalmic genetics 2008;29.2: 47–52
- ↑ Jump up to: 3.0 3.1 3.2 Lingam G, Badrinath SS, Kumar KS, Doshi G, Biswas N. Optic disc in fundus coloboma. Ophthalmology. 1996;103:2120–2126
- ↑ Jump up to: 4.0 4.1 4.2 Brodsky MC. Congenital Optic Disk Anomalies. Survey of Ophthalmology. 1994;39.2:89–112
- ↑ Jump up to: 5.0 5.1 Pollock S. The morning glory disc anomaly: Contractile movement, classification, and embryogenesis. Documenta Ophthalmologica. 1987;65:439-460
- ↑ Jump up to: 6.0 6.1 6.2 Duvall J, Miller SL, Cheatle E, Tso MO. Histopathologic study of ocular changes in a syndrome of multiple congenital anomalies. Am J Ophthalmol. 1987;103:701–705
- ↑ Jump up to: 7.0 7.1 Chu Y, Hughes S, Chan-Ling T. Differentiation and migration of astrocyte precursor cells and astrocytes in human fetal retina: relevance to optic nerve coloboma. FASEB J. 2001;15:2013–2015
- ↑ Ohno-Matsui K, Hirakata A, Inoue M, Akiba M, Ishibashi T. Evaluation of Congenital Optic Disc Pits and Optic Disc Colobomas by Swept-Source Optical Coherence Tomography. Investigative ophthalmology & visual science 2013;54(12): 7769–7778
- ↑ Lingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. Eye (Lond). 2021;35(8):2086-2109
- ↑ Bacci, Giacomo M. MD, PhD; Polizzi, Silvio MD; Mari, Francesco MD, PhD; Conti, Valerio PhD; Caputo, Roberto MD; Guerrini, Renzo MD, FRCP. Atypical Ocular Coloboma in Tuberous Sclerosis-2: Report of Two Novel Cases. Journal of Neuro-Ophthalmology 41(3):p e363-e365, September 2021. | DOI: 10.1097/WNO.0000000000001099
- ↑ Jump up to: 11.0 11.1 11.2 11.3 11.4 11.5 Khan AO, Nowilaty SR. Early diagnosis of the papillorenal syndrome by optic disc morphology. J Neuroophthalmol. 2005;25(3):209-211
- ↑ Jump up to: 12.0 12.1 12.2 Ng B, De Silva S, Bindra M. Papillorenal syndrome: a systemic diagnosis not to be missed on funduscopy. BMJ Case Rep. 2021;14(7):e241708
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 13.8 13.9 Parsa CF, Silva ED, Sundin OH, et al. Redefining papillorenal syndrome: an underdiagnosed cause of ocular and renal morbidity. Ophthalmology. 2001;108(4):738-749
- ↑ Gardner TW, Zaparackas ZG, Naidich TP. Congenital optic nerve colobomas: CT demonstration. J Comput Assist Tomogr. 1984;8:95-102
- ↑ Jump up to: 15.0 15.1 Wise JB, Maclean AL, Gass JD. Contractile peripapillary staphyloma. Arch Ophthalmol 1966;75:626-630
- ↑ Brodsky MC. Contractile morning glory disc causing transient monocular blindness in a child. Arch Ophthalmology. 2006;8:1199-1201
- ↑ Graether JM. Transient amaurosis in one eye with simultaneous dilatation of retinal veins in association with a congenital anomaly of the optic nerve head. Arch Ophthalmology. 1963;70:342-345
- ↑ Jump up to: 18.0 18.1 Hokazono K, Carstens LD, Monteiro RB. Contractile Peripapillary Staphyloma: OCTA Documentation of Increased Peripapillary Vessel Density During Transient Visual Loss Episodes. American journal of ophthalmology case reports. 2021;21
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 Cogan DG. Coloboma of Optic Nerve with Overlay of Peripapillary Retina. Brittish journal of ophthalmology 1978;62(6):347–350
- ↑ Cao XS, Peng XY, You QS, et al. Congenital contractile peripapillary staphyloma with rhegmatogenous retinal detachment. Retin Cases Brief Rep. 2016;12(1):48–49
- ↑ Kunita D, Inoue M, Koto T, Hirakata A. Retinal detachment associated with peripapillary staphyloma imaged with swept source optical coherence tomography. Retin Cases Brief Rep. 2017;13(1):25–29
- ↑ Jump up to: 22.0 22.1 Willis R, Zimmerman LE, O'Grady R, Smith RS, Crawford B. Heterotopic Adipose Tissue and Smooth Muscle in the Optic Disc: Association With Isolated Colobomas. Arch Ophthalmol. 1972;88(2):139–146.
- ↑ Warburg M. Classification of microphthalmos and coloboma. Journal of Medical Genetics 1993;30:664-669
- ↑ Gordon R, Chatfield RK. Pits in the optic disc associated with macular degeneration. The British journal of ophthalmology. 1969;53(7):481–9.
- ↑ Jump up to: 25.0 25.1 Reed D. Congenital pits of the optic nerve. Clin Eye Vis Care. 1999;11(2):75–80
- ↑ Chatziralli I, Theodossiadis P, Theodossiadis GP. Optic disk pit maculopathy: Current management strategies. Clinical Ophthalmology. 2018;12:1417–1422