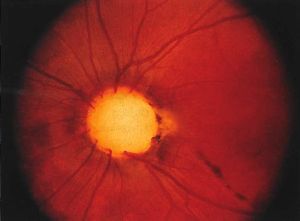
Optic Nerve Coloboma Spectrum
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Overview
Optic Nerve Coloboma Spectrum comprises a spectrum of diseases with overlapping characteristics (cavitation of the optic disc), but overall different clinical findings, etiologies, and associations. Some of the diseases discussed are Optic Disc coloboma, Morning Glory Syndrome, Peripapillary Staphyloma, Pedler Coloboma, and Optic Disc pits.[1]
Nomenclature
The nomenclature regarding the spectrum of diseases can be confusing and include “Optic Disc Cavitary Lesions,” “Excavated Optic Disk anomalies,” and “Optic Nerve Coloboma Spectrum.” In the literature and in this EyeWiki we use the terms “Optic Disc” coloboma and “Optic Nerve” coloboma interchangeably.[1]
Management
Management of optic disc excavations is focused on early detection and visual correction for amblyopia (e.g., the trial of patching or early optimal refraction in optic disc coloboma) and awareness of common associations/complications for proactive management (e.g., neuroimaging in morning glory syndrome for associated basoencepholcele). There is no treatment or prevention for cavitary disc anomalies.[1][2]
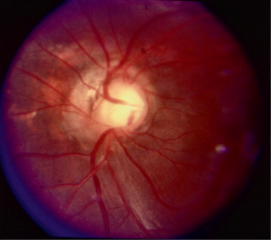
Optic Disc coloboma
Clinical findings/Physical Examination
The optic disc coloboma (ODC) is characterized by a bowl shaped excavation often inferiorly with sharp borders. Unlike the morning glory disc, the ODC has no central glial tuft and the disc vasculature is usually normal. ODC can mimic glaucomatous cupping[1][3] but the excavation in ODC is normally decentered inferiorly and is non-progressive.[4] Microphthalmia can be present if the ODC related excavation includes the retina or choroid. Visual acuity in ODC can be difficult to predict from the appearance of the disc alone and can range from mild to severely affected vision. Unilateral or bilateral ODC may occur.[5]
Etiology
ODC is believed to be the result of incomplete closure of the proximal part of the embryonal fissure during the sixth week of gestation.[6] PAX2 genes, expressed in astrocytic cells, are also expressed in adult retinal cells located on the optic nerve head. Precursor astrocytes are found on the edge of the optic nerve during early development – forming the theory that abnormal astrocyte differentiation or migration during development may play a role in optic nerve coloboma formation.[7]
Ancillary testing
One study of ODC used swept-source optical coherence tomography (OCT) and described sparse and irregularly oriented scleral fibers and openings of the subarachnoid space just posterior to the bottom of the excavation.[8]
Associations
ODC can be associated with a number of genetic disorders (e.g., PAX gene mutations, Walker-Walburg syndrome, Aicardi syndrome). Isolated ODC without systemic abnormalities can be sporadic or familial[9] There is no known racial or sexual predilection in ODC.[3] An important association is papillorenal syndrome. Few isolated cases with both anterior and posterior coloboma have been described in Tuberous sclerosis[10].
Another significant complication associated with optic disc coloboma is serous maculopathy, which requires careful monitoring and management.
Serous maculopathy associated with optic nerve head coloboma
Clinical Features: Enlarged disc with white glistening excavation within it
- Subretinal fluid underneath macula extending from optic nerve
Pathophysiology: Subretinal fluid may arise from
- Fluid entering into the retrobulbar space from surrounding orbital tissue
- Peripapillary choriocapillaris
- CSF
Management: Photocoagulation along temporal margin of disc has been described
Papillorenal syndrome (Renal-coloboma syndrome)
Clinical Findings/PE
Optic disc excavations in the papillorenal syndrome commonly lack central retinal vessels.[11][12] [13] There are usually multiple cilioretinal vessels surrounding the optic disc and arising from its periphery, typically inferotermporally.[11] These can lead to static nonarcuate superonasal field defects with steep contracted isopters with geographic borders.[13] Ocular findings of papillorenal syndrome are commonly mistaken for glaucoma, colobomas, or morning glory anomalies – awareness of the vasculature can help differentiate it from these.[13] Visual acuity is normally unaffected.
Etiology
This syndrome’s alternative name, “renal-coloboma syndrome,” is somewhat of a misnomer as there is no evidence that failure in the closure of the optic fissure is related to its etiology like it is with optic disc coloboma.[13] The common anomaly found in papillorenal syndrome is optic disc dysplasia.[11] [12] [13] This finding is thought to be secondary to abnormal angiogenesis, leading to the compromised growth of the retina and choroid.[13] Similarly to optic disc colobomas, papillorenal syndrome is related to mutations of the PAX2 gene.[11] [12] [13] It’s hypothesized that some PAX2 mutations may lead to abnormal development of the optic stalk resulting in abnormal vascularity on the optic nerve head.[13] One study analyzing PAX2 mutations in 11 families with the syndrome failed to detect PAX2 mutations in 3 of the families; suggesting that genetic heterogeneity (mutations in several other genes responsible for the phenotype) is responsible for some cases.[13]
Associations
Peripheral disc excavations that disrupt glial border tissue can lead to cerebrospinal fluid flow and subsequent serous retinal detachment.[11] PAX2 disruption also leads to abnormal mesenchymal differentiation of renal and urinary tracts – this hypodysplasia can lead to renal hypoplasia, hypertension, and failure.[13] Additionally, patients with papillorenal syndrome have been found to have bilateral neurosensory hearing loss, laxity of the ligaments, and sometimes neurological malformations and epilepsy.[11]
Morning Glory Disc (MGD)
Clinical Findings/PE
The morning glory disc (MGD) is so named because it resembles the morning glory flower. The key ophthalmoscopic features of the MGD are a funnel-shaped excavation with a tuft of glial tissue lying at the center of the excavation and a dramatic and increased retinal vascularity radiating outwards from the periphery of the optic disc.[2] MGD is typically unilateral, and the visual acuity is poor (typically ranging from 20/200 to finger counting).
Etiology
The exact etiology of the MGD is unclear. Still, the most accepted hypothesis suggests that the pathogenesis may be similar to that of ODC with a defective closure of the embryonic fissure. Some authors believe that MGD and ODC are on the same phenotypic spectrum of colobomatous disc defects.[14][5]
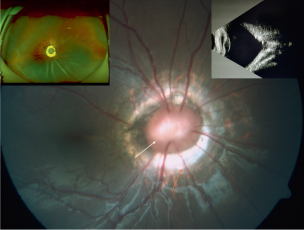
Associations
The classic association of MGD is the basal encephalocele (an outpouching of the meninges). Additional associations with MGD are cerebrovascular anomalies, PHACE (Posterior fossa, Hemangioma, Arterial lesions, Cardiac abnormalities, Eye abnormalities) syndrome, and neurofibromatosis type 2.[4] In contrast to ODC, MGD is more common in females and Caucasians but has rarely been reported in African Americans.[3]
Peripapillary Staphyloma
Clinical Findings/PE
The optic disc usually looks normal in cases of peripapillary staphyloma (PPS). PPS may be unilateral or bilateral with atrophic pigmentary changes.[15] The vascularity of the disc in PPS is normal and differs from the ODC and MGD.[2] Visual acuity can range from markedly reduced to normal depending on the involvement of the macular and those with decreased vision frequently have centrocecal scotomas.[15] Transient vision loss may occur, a recent study suggests that contractions, secondary to either pressure imbalance or muscle contractions, leading to a decrease in vessel density which may be responsible for the transient vision loss. Spontaneous contractile movements of the optic disc region have been seen also in ODC and MGD.[16] [17] [18]
Ancillary testing
The PPS demonstrates on ultrasound, OCT, and fundus exam peripapillary atrophy and excavation with thinning o of the retinal layers around the posterior staphyloma and steep slope to the bottom of the disc.[18]
Etiology
The suggested etiology is that it is due to diminished peripapillary structural support resulting from incomplete differentiation of sclera from posterior neural crest cells at 5 months gestation. Once there is the establishment of normal intraocular pressure the PPS is formed via herniation of unsupported ocular tissues through the defect.[4][19]
Associations/complications
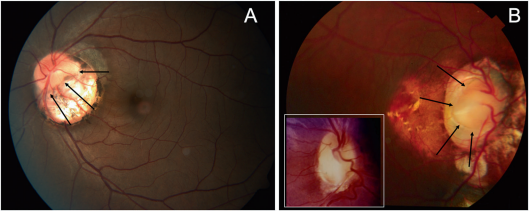
Some case reports have described retinal detachment around the optic disc in eyes with PPS.[20] [21]
Pedler coloboma
Clinical Findings/PE
Described first by Pedler in 1961, Pedler Coloboma (PC) has been described as an optic disc with heterotopic adipose tissue and smooth muscle). The PC presents as an abnormally wide scleral canal filled with connective tissue.[19][22] Buckling of the retina can form an epipapillary mass that may be mistaken for an ocular tumor. The PC is described as loose richly vascularized connective tissue located in the region of the physiological optic cup.[19] The optic disc can be seen synchronously pulsating with respiration.[19][23]
Etiology
PC is believed to result from a failure of maturation in the posterior sclera (secondary to derangement of the optic cup) leading to failed fusion of the peripapillary retina, optic disc, lamina cribrosa, and posterior choroid. These resultant layers are then driven posteriorly through the resultant hiatus.[19]
Associations
Cases including these heterotopic mesodermal characteristics described by Willis et al. were all in association with isolated colobomas.[22]
Histopathological analysis
Commonly a large scleral canal will be seen with absence or defective formation of the lamina cribosa. Glial tissue and hyperplastic pigment epithelium is present between the optic nerve and the sclera.[7]
Optic pit (OP)
Clinical Findings/PE
The optic pit (OP) is a crater-like depression of the optic nerve head.[6] OP has no systemic findings and is commonly found on the temporal side of the disc.[6] Depending on the size of the OP and its location there may or may not be visual field loss (e.g., arcuate field defects) but visual acuity is typically not affected.[24]
Etiology
The etiology of the OP is thought to be similar to ODC and MGD with incomplete closure of the superior edge of the embryonic fissure.[25]
Complications/Associations
Common complications of the OP are serous retinal detachment and/or retinoschisis in the area of the macular, this is called OP maculopathy.[25]
Management and treatment
If the macula is affected with serous detachment in OP then treatment modalities include laser photocoagulation to create a barrier between the disc and the macula so fluid doesn’t enter the macula, pars plana vitrectomy (greater success than photocoagulation), injecting gas tamponade, and/or macular buckle.[26] Treatment of Optic pit with neurosensory detachment is challenging. Newest treatments have proven efficacy with retinal surgery and use of human amniotic membrane[27][28].
Summary
Clinicians should recognize the spectrum of optic disc related cavitary disc anomalies and neuroimaging may be necessary of some cases to exclude associated intracranial defects (e.g., basal encephalocoele).
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Dutton G. Congenital disorders of the optic nerve: excavations and hypoplasia. Eye. 2004;18:1038–1048
- ↑ Jump up to: 2.0 2.1 2.2 Lee BJ, Elias IT. Update on the Morning Glory Disc Anomaly. Ophthalmic genetics 2008;29.2: 47–52
- ↑ Jump up to: 3.0 3.1 3.2 Lingam G, Badrinath SS, Kumar KS, Doshi G, Biswas N. Optic disc in fundus coloboma. Ophthalmology. 1996;103:2120–2126
- ↑ Jump up to: 4.0 4.1 4.2 Brodsky MC. Congenital Optic Disk Anomalies. Survey of Ophthalmology. 1994;39.2:89–112
- ↑ Jump up to: 5.0 5.1 Pollock S. The morning glory disc anomaly: Contractile movement, classification, and embryogenesis. Documenta Ophthalmologica. 1987;65:439-460
- ↑ Jump up to: 6.0 6.1 6.2 Duvall J, Miller SL, Cheatle E, Tso MO. Histopathologic study of ocular changes in a syndrome of multiple congenital anomalies. Am J Ophthalmol. 1987;103:701–705
- ↑ Jump up to: 7.0 7.1 Chu Y, Hughes S, Chan-Ling T. Differentiation and migration of astrocyte precursor cells and astrocytes in human fetal retina: relevance to optic nerve coloboma. FASEB J. 2001;15:2013–2015
- ↑ Ohno-Matsui K, Hirakata A, Inoue M, Akiba M, Ishibashi T. Evaluation of Congenital Optic Disc Pits and Optic Disc Colobomas by Swept-Source Optical Coherence Tomography. Investigative ophthalmology & visual science 2013;54(12): 7769–7778
- ↑ Lingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. Eye (Lond). 2021;35(8):2086-2109
- ↑ Bacci, Giacomo M. MD, PhD; Polizzi, Silvio MD; Mari, Francesco MD, PhD; Conti, Valerio PhD; Caputo, Roberto MD; Guerrini, Renzo MD, FRCP. Atypical Ocular Coloboma in Tuberous Sclerosis-2: Report of Two Novel Cases. Journal of Neuro-Ophthalmology 41(3):p e363-e365, September 2021. | DOI: 10.1097/WNO.0000000000001099
- ↑ Jump up to: 11.0 11.1 11.2 11.3 11.4 11.5 Khan AO, Nowilaty SR. Early diagnosis of the papillorenal syndrome by optic disc morphology. J Neuroophthalmol. 2005;25(3):209-211
- ↑ Jump up to: 12.0 12.1 12.2 Ng B, De Silva S, Bindra M. Papillorenal syndrome: a systemic diagnosis not to be missed on funduscopy. BMJ Case Rep. 2021;14(7):e241708
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 13.8 13.9 Parsa CF, Silva ED, Sundin OH, et al. Redefining papillorenal syndrome: an underdiagnosed cause of ocular and renal morbidity. Ophthalmology. 2001;108(4):738-749
- ↑ Gardner TW, Zaparackas ZG, Naidich TP. Congenital optic nerve colobomas: CT demonstration. J Comput Assist Tomogr. 1984;8:95-102
- ↑ Jump up to: 15.0 15.1 Wise JB, Maclean AL, Gass JD. Contractile peripapillary staphyloma. Arch Ophthalmol 1966;75:626-630
- ↑ Brodsky MC. Contractile morning glory disc causing transient monocular blindness in a child. Arch Ophthalmology. 2006;8:1199-1201
- ↑ Graether JM. Transient amaurosis in one eye with simultaneous dilatation of retinal veins in association with a congenital anomaly of the optic nerve head. Arch Ophthalmology. 1963;70:342-345
- ↑ Jump up to: 18.0 18.1 Hokazono K, Carstens LD, Monteiro RB. Contractile Peripapillary Staphyloma: OCTA Documentation of Increased Peripapillary Vessel Density During Transient Visual Loss Episodes. American journal of ophthalmology case reports. 2021;21
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 Cogan DG. Coloboma of Optic Nerve with Overlay of Peripapillary Retina. Brittish journal of ophthalmology 1978;62(6):347–350
- ↑ Cao XS, Peng XY, You QS, et al. Congenital contractile peripapillary staphyloma with rhegmatogenous retinal detachment. Retin Cases Brief Rep. 2016;12(1):48–49
- ↑ Kunita D, Inoue M, Koto T, Hirakata A. Retinal detachment associated with peripapillary staphyloma imaged with swept source optical coherence tomography. Retin Cases Brief Rep. 2017;13(1):25–29
- ↑ Jump up to: 22.0 22.1 Willis R, Zimmerman LE, O'Grady R, Smith RS, Crawford B. Heterotopic Adipose Tissue and Smooth Muscle in the Optic Disc: Association With Isolated Colobomas. Arch Ophthalmol. 1972;88(2):139–146.
- ↑ Warburg M. Classification of microphthalmos and coloboma. Journal of Medical Genetics 1993;30:664-669
- ↑ Gordon R, Chatfield RK. Pits in the optic disc associated with macular degeneration. The British journal of ophthalmology. 1969;53(7):481–9.
- ↑ Jump up to: 25.0 25.1 Reed D. Congenital pits of the optic nerve. Clin Eye Vis Care. 1999;11(2):75–80
- ↑ Chatziralli I, Theodossiadis P, Theodossiadis GP. Optic disk pit maculopathy: Current management strategies. Clinical Ophthalmology. 2018;12:1417–1422
- ↑ Caporossi T, D'Amico G, Tartaro R, et al. Optic Disk Pit Maculopathy Treatment Using a Human Amniotic Membrane Patch: One-Year Results. Am J Ophthalmol. 2022;240:30-36. doi:10.1016/j.ajo.2022.02.014
- ↑ Rizzo S, Caporossi T, Pacini B, De Angelis L, De Vitto ML, Gainsanti F. Management of Optic Disk Pit-associated Macular Detachment with Human Amniotic Membrane Patch. Retina. 2023;43(1):144-147. doi:10.1097/IAE.0000000000002753