Cherry-Red Spot: Difference between revisions
No edit summary |
m (Text replacement - "== Primary prevention ==" to "== Primary Prevention ==") |
||
| (44 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{Article | {{Article | ||
|Authors= | |Authors=Marianna.Kavalaraki | ||
|Category=Retina/Vitreous | |Category=Articles,Retina/Vitreous | ||
|Assigned editor= | |Assigned editor=Avni.Finn | ||
|Reviewer= | |Reviewer=Avni.Finn | ||
|Date reviewed= | |Date reviewed=November 10, 2024 | ||
|Article status= | |Article status=Up to Date | ||
}} | }} | ||
Cherry red spot is a significant fundoscopic finding in the macula, observed in central retinal | Cherry red spot is a significant fundoscopic finding in the macula, observed in central retinal artery occlusion (CRAO) and a variety of lipid storage disorders. The emphasis on this page is on CRAO, for which the presence of cherry red spot portends worse visual prognosis. | ||
= Disease Entity = | = Disease Entity = | ||
== Disease == | == Disease == | ||
Cherry | [[File:Cherryspot.jpg|thumb|379x379px|Cherry red spot in patient with central retinal artery occlusion.<ref>https://commons.wikimedia.org/wiki/File:Cherry_red_spot_in_patient_with_central_retinal_artery_occlusion_(CRAO).jpg</ref>]] | ||
Cherry red spot at the macula is a clinically significant sign observed on fundus examination in a variety of pathological conditions<ref name=":0">Suvarna J C, Hajela S A. Cherry-red spot. J Postgrad Med 2008;54:54-7</ref>, including retinal infarction, retinal ischemia, and several lysosomal storage disorders.<ref name=":1">Tripathy K, Patel BC. Cherry Red Spot. [Updated 2021 Feb 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: <nowiki>https://www.ncbi.nlm.nih.gov/books/NBK539841/</nowiki></ref> The term refers to the appearance of a red-tinted region at the center of the macula surrounded by retinal opacification<ref name=":1" />, usually present due to the thickening and loss of transparency of the posterior pole of the retina.<ref name=":2">General Practice Notebook</ref> The fovea retains relative transparency as it is the thinnest part of the retina and devoid of ganglion cells. The fovea receives its blood supply from the choroid, which is supplied by the long and short posterior ciliary arteries.<ref>USMLE First AID 2010 page 417</ref> The appearance of a cherry red spot results when retinal edema, most commonly due to central retinal artery occlusion or traumatic retinal ischemia, causes the perimacular tissue of the retina to become less transparent, while the fovea maintains its normal color.<ref name=":3">Leavitt, J. A., & Kotagal, S. (2007). ''The “Cherry Red” Spot. Pediatric Neurology, 37(1), 74-75 ''doi:10.1016/j.pediatrneurol.2007.04.011 </ref><ref name=":2" /> In the case of lipid storage diseases, the lipids are stored in the ganglion cell layer of the retina, giving the retina a white appearance. As ganglion cells are absent at the foveola, this area retains relative transparency, in contrast to the surrounding retina.<ref name=":2" /> Cherry red spot is an important fundoscopic sign, and when found with key clinical features and a good history, often guides one to the diagnosis of the disease.<ref name=":0" /> It should be noted that as a condition resolves or evolves over the course of time, the appearance of a cherry red spot diminishes and may not be present; therefore, absence of a cherry red spot does not necessarily indicate absence of disease.<ref name=":1" /> | |||
== History == | == History == | ||
The term “cherry | The term “cherry red spot” was first introduced in 1887 by American neurologist Bernard Sachs, who described the fundus appearance of a child with "amaurotic familial idiocy" in his publication on “arrested development with special reference to its cortical pathology”. <ref name=":3" /> The patient was confirmed upon neuropathologic examination to have lipid storage in the brain. <ref>Leavitt, J. A., & Kotagal, S. (2007). The “Cherry Red” Spot''. Pediatric Neurology, 37(1), 74–75.'' doi:10.1016/j.pediatrneurol.2007.04.011 </ref> Bernard Sachs acknowledged that his patient had also been examined by ophthalmologist Herman Joseph Knapp, who practiced in both New York and Berlin, and described the striking retinal findings at an ophthalmology meeting in Heidelberg using the term “cherry red color” to describe the fovea.<ref name=":3" /> Knapp initially believed that the cherry red spot was a benign fundoscopic finding, but later realized its grave implications. <ref name=":3" /> | ||
== Etiology == | == Etiology == | ||
The causes of cherry | The causes of cherry red spot in the macula can be categorized by etiology: | ||
==== | ==== Vascular ==== | ||
* Central | * '''[[Retinal Artery Occlusion|Central Retinal Artery Occlusion (CRAO)]]''' - This disease entity characteristically presents as a sudden onset of painless, unilateral vision loss in an elderly patient. Cherry red spot has been found to be present on the initial examination in 90% of permanent CRAO cases and prognosticates poorer visual recovery. <ref name=":1" /> CRAO is classified as arteritic and non-arteritic, which will further guide the management discussed below. <br />CRAO is most commonly (95% of cases) the '''non-arteritic''' '''CRAO''', which is caused by atherothrombosis, thromboembolism, or vasospasm which occludes the central retinal artery. <ref name=":1" /> Thrombus or thromboembolism may be composed of cholesterol, fibrin-platelet, or calcium; they usually originate from the carotid plaque and less commonly from the heart or the aorta. Associations with thromboembolic causes of CRAO include myxoma or vegetations of the cardiac valves and hypercoagulable states (hyperhomocysteinemia, factor V Leiden, protein C and S deficiencies, anti-thrombin III deficiencies, anti-phospholipid antibodies or prothrombin gene mutations, sickle cell disease). Non-thromboembolic vascular causes of CRAO include nocturnal arterial hypotension, retinal migraine, and transient vasospasm. <ref name=":7">Kaufman EJ, Mahabadi N, Patel BC. Hollenhorst Plaque. [Updated 2021 Feb 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: <nowiki>https://www.ncbi.nlm.nih.gov/books/NBK470445/</nowiki></ref><ref name=":8">Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013 Jun;27(6):688-97. doi: 10.1038/eye.2013.25. Epub 2013 Mar 8. PMID: 23470793; PMCID: PMC3682348.</ref> '''Arteritic''' '''CRAO''' occurs in the setting of inflammatory conditions like [[Giant_Cell_Arteritis|giant cell arteritis (GCA)]] and are an estimated 5% of CRAO cases. Other inflammatory disorders are less common causes of arteritic CRAO and include Susac syndrome, systemic lupus erythematosus, polyarteritis nodosa, and granulomatosis with polyangiitis.<ref name=":4">Sim, S., & Ting, D. (2017, August). ''Diagnosis and Management of Central Retinal Artery Occlusion''. American Academy of Ophthalmology. <nowiki>https://www.aao.org/eyenet/article/diagnosis-and-management-of-crao</nowiki>.</ref> | ||
* Acute occlusion of the | * Acute occlusion of the retinal circulation following cataract surgery (phacoemulsification) causing outer retinal whitening. <ref>Central retinal artery occlusion after phacoemulsification under peribulbar anaesthesia: S Rodríguez Villa,R Salazar Méndez,M Cubillas Martín,M Cuesta García. Eur J Ophthalmol. 2021 Mar;31(2):NP77 PMID: '''26652970''' DOI: 10.1016/j.oftal.2015.10.003 </ref> | ||
* | * Compromise of the choroidal and retinal circulation due to intraorbital hemorrhage or mass. (Note that an ophthalmic artery occlusion may not manifest a cherry red spot due to compromise of both the choroidal and retinal circulations.) | ||
* Macular infarction due to various | * Macular infarction due to various causes such as retinal artery and vein occlusions, carotid artery occlusions, malignant hypertension, diabetes, radiation retinopathy, posterior uveitis, systemic inflammatory vasculopathies, and trauma. <ref>Gass J.D.M. A fluorescein angiographic study of macular dysfunction secondary to retinal vascular disease. Arch Ophthalmol. 1968;80:535–617.</ref> <ref>Bentley C.R., Stanford M.R., Shilling J.S., Sanders M.D., Graham E.M. Macular ischaemia in posterior uveitis. Eye (Lond) 1993;7:411–414.</ref><ref>Acacio I., Goldberg M.F. Peripapillary and macular vessel occlusions in sickle cell anemia. Am J Ophthalmol. 1973;75:861–866.</ref><ref>Goel N, Rajput M, Sawhney A, Sardana T. Macular infarction and traumatic optic neuropathy following blunt ocular trauma. Saudi J Ophthalmol. 2016;30(1):53-55. doi:10.1016/j.sjopt.2015.08.002</ref> | ||
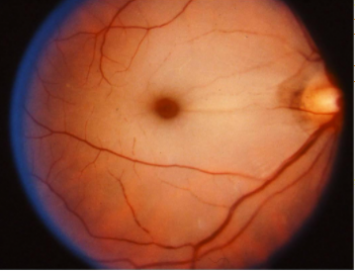
[[File:Taysacks.PNG|thumb|355x355px|Prominent cherry-red spot in a patients with Tay-Sachs disease.<ref>Learning About Tay-Sachs Disease [Internet]. 2011 [cited 2013 Oct 20] Available from: <nowiki>http://www.genome.gov/10001220</nowiki></ref>]] | |||
==== | ==== Metabolic Storage Diseases ==== | ||
* Tay-Sachs disease ( | * GM 2 gangliosidosis – a group of autosomal-recessive disorders with deficiencies in lysosomal enzymes beta-hexosaminidase (HEX), including Tay-Sachs disease (beta hexoaminidase A deficiency) and Sandhoff (beta hexoaminidase A+B). New evidence suggests different disease cohorts with some presenting with early onset and rapid disease progression and others with later symptom onset and variable disease progression. There is an abnormal accumulation of gangliosides in the brain and ganglion cell layer of the retina. A cherry red spot is present in approximately 75-90% of the patients affected with early-onset disease. <ref>Kern J, Böhringer J, Timmann D, et al.. Clinical, Imaging, Genetic, and Disease Course Characteristics in Patients With GM2 Gangliosidosis. Neurology. 2024; 102 (1): doi: 10.1212/WNL.0000000000207898</ref> | ||
* Niemann-Pick disease - Type A, B, C, D disease | * Niemann-Pick disease - Type A, B, C, D disease | ||
* GM1 gangliosidosis type 1 (Generalized gangliosidosis or Landing disease) | * GM1 gangliosidosis type 1 (Generalized gangliosidosis or Landing disease) - an autosomal recessive disorder caused by mutations in ''GLB1'' in which a cherry-red spot in the macula occurs in around 50% cases | ||
* Sialidosis or mucolipidosis type 1 | * Sialidosis or mucolipidosis type 1 - a lysosomal storage disease caused by alpha-neuraminidase deficiency (''NEU1'' gene mutation) which presents with ataxia, movement disorders, nystagmus, and myoclonic seizures. Intellect is usually normal, and somatic features are generally not seen. Two types have been described according to the age of onset and severity of the disease: Sialidosis type I or cherry red spot myoclonus syndrome is characterized by onset in the 2nd or 3rd decade, gait disturbances, myoclonus, ataxia, tremor, a cherry red spot at the macula, and seizures. The intellect and life expectancy are usually unaffected, though the patient eventually needs wheelchair assistance with time. Almost all the patients have a cherry red spot at the macula. | ||
* Farber disease (Farber lipogranulomatosis) | * Farber disease (Farber lipogranulomatosis) - a rare autosomal recessive (AR) disorder characterized by Ceramidase or acid ceramidase (chromosome 8p22) deficiency, affecting the ''GLA'' gene . The typical triad consists of painful swollen joints, subcutaneous nodules (lipogranulomas), and weak cry or hoarse voice due to laryngeal nodules. Other features include a developmental delay, visceromegaly, respiratory involvement, and neurological features, including quadriplegia, seizures, and myoclonus. | ||
* Metachromatic leukodystrophy | * Metachromatic leukodystrophy - another rare (AR) lysosomal storage disorder described by arylsulfatase A deficiency, resulting in abnormal collection of sulfatides in myelin-producing cells that leads to progressive destruction of white matter (leukodystrophy). A cherry red spot at the macula is occasionally found, along with progressive decline in intellectual and motor functions, peripheral neuropathy resulting in diminished sensations, hearing loss, incontinence, loss of speech and mobility, blindness, unresponsiveness, and eventual death. | ||
* Galactosialidosis (Goldberg Cotlier syndrome or neuraminidase deficiency with beta-galactosidase deficiency or cathepsin A deficiency) is an AR disorder due to a mutation in the CTSA gene coding for cathepsin A. Ocular features include a cherry | * Galactosialidosis (Goldberg Cotlier syndrome or neuraminidase deficiency with beta-galactosidase deficiency or cathepsin A deficiency) is an AR disorder due to a mutation in the ''CTSA'' gene coding for cathepsin A. Ocular features include a cherry red spot at the macula, corneal clouding, and conjunctival telangiectasia. | ||
==== | ==== Inflammation ==== | ||
* Retinitis involving the central retina, including progressive outer retinal necrosis | * Retinitis involving the central retina, including progressive outer retinal necrosis and subacute sclerosing panencephalitis. <ref name=":1" /> | ||
==== | ==== Drug Toxicity ==== | ||
* Quinine | * Quinine | ||
| Line 61: | Line 61: | ||
* Intravitreal gentamicin | * Intravitreal gentamicin | ||
==== | ==== Trauma ==== | ||
* Commotio | * [[Commotio Retinae]] - Acute retinal opacification following blunt trauma, also known as Berlin's edema, can cause a pseudo-cherry red spot, as a result of peripheral whitening of the retina that involves the macula. | ||
* Optic | * [[Optic_Nerve_Head_Avulsion|Optic Nerve Avulsion]] <ref>Sudden occlusion of the retinal and posterior choroidal circulations in a youth. | ||
Brown GC, Magargal LE.Brown GC, et al. Am J Ophthalmol. 1979 Oct;88(4):690-3. doi: 10.1016/0002-9394(79)90666-4.</ref> | |||
* [[Saturday Night Retinopathy]] - Occlusion of the retinal or ophthalmic artery due to headrest in a prolonged prone body position which results in self-induced sustained direct orbit compression and collapse of orbital vessels. A cherry red spot may appear if circulation in the choroid remains present.<ref>Self-induced Orbital Compression Injury: '''Saturday''' '''Night''' Retinopathy. | |||
Williams AL, Greven M, Houston SK, Mehta S.Williams AL, et al. JAMA Ophthalmol. 2015 Aug;133(8):963-5. doi: 10.1001/jamaophthalmol.2015.1114.</ref><ref>Williams AL, Greven M, Houston SK, Mehta S. Self-induced Orbital Compression Injury: Saturday Night Retinopathy. JAMA Ophthalmol. 2015;133(8):963–965. doi:10.1001/jamaophthalmol.2015.1114</ref> | |||
* Leukemic infiltrates of the optic nerve head may cause CRAO and cherry red spot | * Leukemic infiltrates of the optic nerve head may cause CRAO and cherry red spot | ||
== Risk Factors == | == Risk Factors == | ||
Risk factors for cherry red spot due to non-arteritic CRAO are similar to those for acute ischemic stroke: diabetes, arterial hypertension, transient ischemic attack, coronary artery disease, smoking, and vascular disease. For retinal artery occlusion in particular, internal carotid artery stenosis is commonly associated, with a prevalence as high as 70% reported among patients with CRAO or branch retinal artery occlusion.<ref>Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002;33:1963–1967. doi: 10.1161/01.str.0000023445.20454.a8</ref> | |||
Please refer to above section on etiologies for common associations with arteritic CRAO or other causes of cherry red spot. | |||
== Pathophysiology == | == Pathophysiology == | ||
The macula is histologically characterized by an area consisting of more than one layer of ganglion cell layers, | The macula is histologically characterized by an area consisting of more than one layer of ganglion cell layers, so the ganglion cell layer is thicker at the macula. In disease entities that cause transparency loss (whitening or opacification) of the inner retina, the reddish color of the vascular choroid and pigmentation of retinal pigment epithelium (RPE) is not visible through the opacified retina. However, the foveola is devoid of the inner retinal layer. The retinal layers present at the foveola are (from vitreal to the sclera)- internal limiting membrane, outer nuclear layer, external limiting membrane, photoreceptor layer, and RPE. Inner retinal layers are absent in mature foveola. The vascular supply of the fovea is from the choroid and is not affected by occlusion of retinal vessels. Thus, the foveola, the thinnest part of the central retina, does not lose its transparency during inner retinal ischemia. | ||
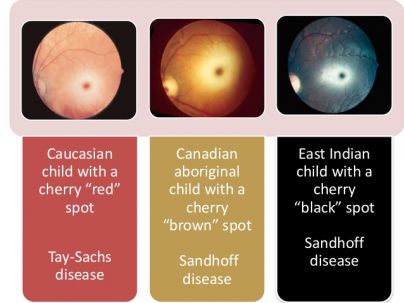
Thus, when there is inner retinal opacification, the reddish color of the vascular choroid and the RPE | Thus, when there is inner retinal opacification, the reddish color of the vascular choroid and the RPE remain visible through the foveola, which is surrounded by an area of the white/opacified retina, giving rise to the typical cherry red spot. As described above, causes of opacification of the inner layers of the retina include compound deposition, as in storage diseases, and ischemia from vascular compromise involving the retinal but not the underlying choroidal circulation.[[File:Varients.jpg|thumb|404x404px|Variants of the "cherry red spot' sign in different ethnicities.]] | ||
The size of the cherry red spot depends on the size of the foveola. As the color of the choroid and RPE varies with race, the color of the foveola too may vary by race. In Caucasian individuals, the foveola appears red, whereas in non-Caucasian patients; the color of foveola may vary and has been reported to be brown in a Canadian aboriginal child with Sandhoff disease and black in a patient of East Indian descent with Sandhoff disease. Thus, the alternate term ‘perifoveal white patch’ has been suggested. Note that when the ''ophthalmic'' artery is occluded, compromise to the vascular supply of the retina, choroid, and optic nerve head circulation leaves retinal opacification ''without'' a cherry red spot and the patient experiences severe vision loss with loss of light perception. | |||
Opacification of the inner retina can occur as a result of retinal ischemia due to occlusion of the central retinal artery. The retinal whitening is typically most prominent in the posterior pole, and the opaque retina obscures the details of the choroidal vessels. The whitening of the retina becomes less prominent a little peripheral to the arcades, which may be related to the reduced inner retinal thickness at the peripheral retina. There is a cessation of axoplasmic transport and then the opacification of both the ganglion cell and the nerve fiber layer in a CRAO. | |||
A variety of lipid storage disorders can produce deposition / accumulation of material at the level of the ganglion cell layer can other lipid storage disorders (sphingolipidoses), leading to progressive abnormal excessive intracellular accumulation of various glycolipids/phospholipids in the ganglion cell layer, that cause opacification of the inner retina. The foveola is devoid of the ganglion cell layer and hence remains transparent and allows transmission of the color of the choroid and the RPE. However, with increasing age, the damaged ganglion cells are lost, and the cherry red spot becomes less prominent. In such cases, there is consecutive optic atrophy and pallor of the optic disc due to the degeneration of the ganglion cell layer and nerve fiber layer. | |||
== Primary Prevention == | |||
Prevention for retinal ischemia in non-metabolic causes of cherry red spot include measures to reduce cardiovascular and cerebrovascular co-morbidities. | |||
Prevention for retinal ischemia | |||
= Diagnosis = | = Diagnosis = | ||
Ruling out serious life-threatening or sight-threatening conditions is | Ruling out serious life-threatening or sight-threatening conditions is paramount in a patient who presents with a cherry red spot at the macula. Thorough evaluation is warranted, including attention to blood pressure.<ref name=":1" /> Especially in the absence of clinical suspicion for alternative etiologies of cherry red spot (metabolic disease, recent surgery or trauma, drug use – see history below), acute loss of vision with a cherry red spot should raise concern for stroke since CRAO is an ocular variant of acute ischemic stroke. Hence, depending on institution, a stroke or neurology team may need to be consulted. | ||
== History == | == History == | ||
* Preceding history of amaurosis fugax (transient vision loss lasting seconds to minutes, but that may last up to 2 hours) | * Preceding history of [[Amaurosis Fugax (Transient Vision Loss)|amaurosis fugax]] (transient vision loss lasting seconds to minutes, but that may last up to 2 hours) | ||
* Medical history of cardiovascular disease, thromboembolic | * Medical history of known cardiovascular risk factors (obesity, hypertension, diabetes, hypercholesterolemia), prior stroke or cerebrovascular disease, atrial fibrillation, or inherited or acquired thromboembolic states such as hyperhomocystenemia, factor V Leiden, protein C and S and anti-thrombin deficiencies, anti-phospholipid antibodies or prothrombin gene mutations, sickle cell disease, or neoplastic disease | ||
* | * Known family or personal history of systemic inflammatory or autoimmune disease | ||
* Tobacco use | |||
* Family history of lipid/lysosomal storage disorders or metabolic disease | * Family history of lipid/lysosomal storage disorders or metabolic disease | ||
| Line 109: | Line 111: | ||
== Signs == | == Signs == | ||
[[File:Cattle.PNG|thumb|Color fundus photograph of the right eye showing acute CRAO with cherry red spot and cattle trucking of the arterioles.<ref>Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013 Jun;27(6):688-97. doi: 10.1038/eye.2013.25. Epub 2013 Mar 8. PMID: 23470793; PMCID: PMC3682348</ref>]] | |||
Fundoscopic features that present along with cherry red spot at the macula include: | |||
* Yellow-white opacified appearance of the retina in the posterior pole, due to ischemic necrosis affecting the inner half of the retina, which vanishes in 4-6 weeks. | |||
* Cloudy swelling of the retina | |||
* Attenuated retinal arteries | |||
* Thinning, dilation or normal appearance of retinal veins | |||
* Slow segmental blood flow, known as “boxcarring” (“cattle trucking”) of the retinal arteries in severe obstruction.<ref name=":8" /> | |||
* Pallor of the optic disc (39% of CRAO cases) in later stages, as well as visible absence of nerve fiber layer in the region of optic disc<ref name=":5">Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina. 2007 Mar;27(3):276-89. doi: 10.1097/01.iae.0000238095.97104.9b. PMID: 17460582.</ref> | |||
* Presence of emboli (20-40% of CRAO cases) | |||
* | * Pigmentary changes indicate that choroidal circulation is also involved.<ref name=":5" /> | ||
* Presence of a Hollenhorst plaque, which is a clinical finding in cases of central retinal artery occlusion, or one of its branches (BRAO) caused by a lodging embolus deriving from a cholesterol plaque. <ref name=":7" /> | |||
Additional ophthalmic signs concerning for acute CRAO include: | |||
* | * Ipsilateral relative afferent pupillary defect | ||
* Variable degrees of visual loss | |||
* Impaired color vision proportional to impairment in visual acuity | |||
== Symptoms == | == Symptoms == | ||
* Sudden onset painless visual | * Sudden onset of painless monocular vision loss (visual acuity and/or peripheral vision) occurring over several seconds. Degree of visual loss may be variable. | ||
* Symptoms of giant cell arteritis in older patients | * Symptoms of giant cell arteritis in older patients: new-onset headache, jaw claudication, scalp or posterior neck tenderness, proximal muscle and joint aches, anorexia, weight loss, fever | ||
* | * Transient migraine due to vasospasm | ||
* Neurologic symptoms indicative of lysosomal storage disorders or metabolic disease. | |||
== Clinical Diagnosis == | == Clinical Diagnosis == | ||
* Spectral- | * '''Spectral-Domain Optical Coherent Tomography (OCT)''' has been proposed as one modality that might be used to diagnose and monitor CRAO. In CRAO, there is an observed increase in intensity of inner retinal layers compared with age-matched controls, corresponding to the layers supplied by central retinal arteries.<ref name=":9">Chen H, Chen X, Qiu Z, Xiang D, Chen W, Shi F, et al. Quantitative analysis of retinal layers' optical intensities on 3D optical coherence tomography for central retinal artery occlusion. Sci Rep. 2015 Mar 18. 5:9269. | ||
</ref> OCT can be correlated with visual prognosis.<ref name=":9" /> Incomplete CRAO shows minimal retinal architectural disruption and inner layer hyper-reflectivity without retinal edema. Subtotal CRAO demonstrates inner macular thickening and loss of organization of the inner retina, and total CRAO demonstrates marked inner retinal thickening and subfoveal choroidal thinning. <ref>Chen H, Chen X, Qiu Z, Xiang D, Chen W, Shi F, et al. Quantitative analysis of retinal layers' optical intensities on 3D optical coherence tomography for central retinal artery occlusion. ''Sci Rep''. 2015 Mar 18. 5:9269. </ref><ref>Mehta N, Marco RD, Goldhardt R, Modi Y. Central Retinal Artery Occlusion: Acute Management and Treatment. Curr Ophthalmol Rep. 2017 Jun. 5 (2):149-159.</ref> In the chronic phase, there is a corresponding thinning of the inner retinal layers. | |||
* '''Fluorescein Angiography''' may be a prognostic test in CRAO, as poor perfusion on fluorescein angiography has been associated with lower vision than exudative and mixed perfusion. However, this finding does not influence therapy.<ref name=":10">Gong H, Song Q, Wang L. Manifestations of central retinal artery occlusion revealed by fundus fluorescein angiography are associated with the degree of visual loss. Exp Ther Med. 2016 Jun. 11 (6):2420-2424.</ref> Normal choroidal filling begins 1-2 seconds before retinal filling and is complete within 5 seconds of dye appearance in healthy eyes.<ref name=":10" />A delay of 5 or more seconds is seen in 10% of patients.<ref name=":10" /> Ophthalmic artery occlusion or carotid artery obstruction should be considered if choroidal filling is significantly delayed. A delay is noted in arteriovenous transit time (reference range, < 11 seconds), as well as a delay in retinal arterial filling. Arterial narrowing with normal fluorescein transit is observed after recanalization.<ref name=":10" /> | |||
* '''Optical Coherent Tomography Angiography''' provides structural and functional (blood flow) information at a fixed point but is not useful to appreciate leakage from vessels.<ref name="mathew29">Mathew R, Papavasileiou E, Sivaprasad S. Autofluorescence and high-definition optical coherence tomography of retinal artery occlusions. Clin Ophthalmol. 2010 Oct 21. 4:1159-63.</ref> OCTA shows decreased vascular perfusion in superficial and deep retinal plexus that corresponds to poor perfusion on fluorescein angiography.<ref>de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015. 1:5. | |||
</ref> However, unlike fundus fluorescein angiography, OCTA cannot demonstrate a delay in transit time. <ref>Bonini Filho MA, Adhi M, de Carlo TE, Ferrara D, Baumal CR, Witkin AJ, et al. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN RETINAL ARTERY OCCLUSION. Retina. 2015 Nov. 35 (11):2339-46.</ref> | |||
* Fundus | * '''Fundus Autofluorescence''': in the acute phase of CRAO, fundus autofluorescence in ischemic areas is decreased because of retinal edema blocking the normal RPE. Eventually, this could return to normal baseline or may be associated with increased autofluorescence owing to a window defect created by the thinned-out inner retinal layers. <ref name="mathew29"/> | ||
* Electroretinography shows a diminished b-wave corresponding to Muller and/or bipolar cell ischemia. | * '''Electroretinography''' shows a diminished b-wave corresponding to Muller and/or bipolar cell ischemia. | ||
== Diagnostic procedures == | == Diagnostic procedures == | ||
* Blood pressure and pulse monitoring, particularly to detect atrial fibrillation | * Blood pressure and pulse monitoring, particularly to detect atrial fibrillation, possibly with Holter monitor | ||
* ECG to | * Electrocardiogram (ECG) to look for arrhythmia (in particular atrial fibrillation) and other cardiac disease | ||
* Echocardiography | * Echocardiography if there is a specific indication such as a history of rheumatoid fever or known cardiac valvular disease. | ||
* Chest | * Chest x-ray to investigate for sarcoidosis, tuberculosis, left ventricular hypertrophy in hypertension | ||
* Magnetic | * Magnetic Resonance Imaging (MRI), as approximately 20% of patients with a CRAO also have cerebral ischemia; therefore a brain MRI may reveal concurrent cerebral ischemia in patients without accompanying neurological symptoms.<ref>Dattilo M, Biousse V, Newman NJ. Update on the Management of Central Retinal Artery Occlusion. Neurol Clin. 2017 Feb. 35 (1):83-100. | ||
* Magnetic | </ref> | ||
* Computerized tomography (CT) or | * Magnetic Resonance Angiography (MRA) of the head and neck may be more accurate in detecting vascular occlusive disease. | ||
* Computerized tomography (CT) for rapid stroke evaluation or Computerized Tomography Angiography (CT/CTA) or MRI/MRA of the neck if suspecting carotid disease or dissection | |||
* Carotid Doppler Ultrasonography to evaluate for atherosclerotic plaque and arterial flow if suspecting occlusive carotid disease | |||
== Laboratory test == | == Laboratory test == | ||
* Complete blood count CBC (anemia, leukemia, polycythemia, platelet disorders) | * Complete blood count CBC (anemia, leukemia, polycythemia, platelet disorders) | ||
* urea and electrolytes | * Basic metabolic panel to check urea and electrolytes | ||
* Erythrocyte | * Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP) to screen for giant cell arteritis in elderly patients | ||
* | * Hypercoagulablity work-up (e.g, factor V Leiden, prothrombin mutation, homocysteine levels, fibrinogen, anti-phospholipid antibodies, prothrombin time/activated partial thromboplastin time [PT/aPTT]) if suspicious for coagulopathy | ||
* Plasma protein electrophoresis if suspicious for sickle cell disease | |||
* | * Fasting blood sugar, cholesterol, triglycerides, and lipid panel to evaluate risk factors for atherosclerotic disease | ||
* Blood cultures if suspecting bacterial endocarditis and septic emboli | |||
* Antibodies for suspected vasculitidies or rheumatologic disease | |||
* Genetic testing, molecular analysis of cells or tissues, and enzyme assays if considering lipid-storage disorders in younger patients | |||
== Differential diagnosis == | |||
A macular hemorrhage or hole, with resulting enhanced red contrast relative to the surrounding retina, may be confused with a cherry red spot.<ref name=":3" /> | |||
= | Other causes of acute painless loss of vision include vitreous and chorioretinal hemorrhage, retinal detachment, and acute optic neuropathy. | ||
= Management = | |||
Cherry red spot at the macula is a clinically significant fundoscopic finding that usually denotes an ocular emergency, especially in the patient who presents with sudden, painless unilateral vision loss and absence of other preceding clinical factors. The general management of a cherry red spot sign is determined by the underlying pathological condition that causes the appearance of the fundoscopic finding. | |||
In many institutions, suspicion of CRAO, a form of acute ischemic stroke, warrants urgent neurology consultation for stroke work-up.<ref>Terao R, Fujino R, Ahmed T. Risk Factors and Treatment Strategy for Retinal Vascular Occlusive Diseases. J Clin Med. 2022 Oct 27;11(21):6340. doi: 10.3390/jcm11216340. PMID: 36362567; PMCID: PMC9656338.</ref>In addition to appropriate stroke work-up and fundoscopic exam, the patient should be screened for arteritis if in the appropriate demographic. | |||
For metabolic storage disorders, management requires a team of various specialties. Generalized pearls for the management of such patients include avoiding/reducing intake of specific molecules that lead to accumulation within ganglion cells, enzyme replacement therapy, and symptomatic management. | |||
== General treatment == | == General treatment == | ||
Treatment may vary depending on etiology but given the relative frequency and clinical significance of CRAO, discussion here will focus on treatment of CRAO. Please also refer to the page on [[Retinal Artery Occlusion]] for further details. | |||
In the acute setting of CRAO | In the acute setting of CRAO, treatment options are directed at resolving the CRAO and circulation, and thus maximizing visual outcome. Experimental studies suggest no detectable optic nerve or retinal damage in primate models with CRAO if retinal blood flow is restored within 90 minutes. Subsequent partial recovery may be possible if ischemia is reversed within 240 minutes. However, occlusions lasting longer than 240 minutes produce irreversible damage.<ref>Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion: retinal survival time. Exp Eye Res. 2004;78:723–736. doi: 10.1016 s0014-4835(03)00214-8</ref><ref>Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol. 2000;129:786–795. doi: 10.1016/s0002-9394(00)00384-6</ref> The treatment approach is attempted within the first 24 hours. | ||
For various storage diseases, the management requires a team of various specialties. Generalized pearls for the management of such patients include avoiding/reducing intake of specific molecules, enzyme replacement therapy, and symptomatic management. | For various storage diseases, the management requires a team of various specialties. Generalized pearls for the management of such patients include avoiding/reducing intake of specific molecules that lead to accumulation within ganglion cells, enzyme replacement therapy, and symptomatic management. | ||
== Medical therapy == | == Medical therapy == | ||
| Line 206: | Line 223: | ||
==== IV methylprednisolone ==== | ==== IV methylprednisolone ==== | ||
==== Hyperbaric oxygen therapy ==== | ==== Hyperbaric oxygen therapy ==== | ||
== Procedures / Surgery == | == Procedures / Surgery == | ||
* Ocular massage : | * Ocular massage: an attempt to mechanically dislodge the obstructing embolus using in and out movement with the use of a three-mirror (Goldmann) contact lens or digital massage. Pressure should be applied for 10-15 seconds followed by sudden release. Ocular massage can cause arterial dilatation and improve retinal perfusion by an 86% increase in flow volume.<ref name=":4" /> | ||
* Anterior chamber paracentesis : | * Anterior chamber paracentesis: provides sudden decrease in intraocular pressure, and aids the perfusion pressure behind the obstruction to dislocate the obstructing embolus.<ref name=":4" /> | ||
* Transluminal Nd:YAG laser embolysis : | * Transluminal Nd:YAG laser embolysis: this procedure has been advocated for CRAO or BRAO, in cases when the occluding embolus is visible. Shots of 0.5–1.0 mJ or higher are applied directly to the embolus using a fundus contact lens, in order to lyse or dislodge the occluding embolus. <ref name=":4" /> Embolectomy has been said to occur if the embolus is ejected into the vitreous via a hole in the arteriole. The main complication is vitreous hemorrhage. | ||
* Pars plana vitrectomy : Surgical removal of the clot | * Pars plana vitrectomy: Surgical removal of the clot | ||
== Complications == | == Complications == | ||
Neovascularization is a complication that may occur in patients with CRAO with an incidence that varies from 3.0-18.8%. It may involve the retina, iris, or iridocorneal angle. Moreover, the detection of | Neovascularization is a complication that may occur in patients with CRAO with an incidence that varies from 3.0-18.8%. It may involve the retina, iris, or iridocorneal angle. Moreover, the detection of neovascularization following CRAO has ranged from as early as the day of presentation to 2 years after the CRAO diagnosis. The association of CRAO and the development of neovascular glaucoma is thought to occur in about 5%; however, a prospective study established a temporal relationship between CRAO and neovascular glaucoma in 15% of cases. These results are corroborated by studies supporting the occurrence around 8 weeks from diagnosis to clinically evident neovascularization.<ref name=":8" /> However, a prospective study of 232 eyes found neovascularization in 2.5% of cases, and the authors found that there was no causal relationship.<ref name=":6">Jung YH, Ahn SJ, Hong JH, et al. Incidence and Clinical Features of Neovascularization of the Iris following Acute Central Retinal Artery Occlusion. ''Korean J Ophthalmol''. 2016;30(5):352-359. doi:10.3341/kjo.2016.30.5.352</ref> | ||
With frequent visits, many cases of | With frequent visits, many cases of neovascularization can be managed early with treatments such as panretinal photocoagulation to decrease retinal oxygen demand and off-label intravitreal bevacizumab. Since neovascularization can occur early, regular follow-up appointments should be required, especially within the first 4 months.<ref name=":6" /> | ||
== Prognosis == | == Prognosis == | ||
The initial management approach described above may provide some benefit; however, there is no clear evidence at this point that these measures result in improvement of the patient's final visual acuity and have the best chance when performed shortly after the diagnosis of CRAO. Vision loss is permanent due to infarct of inner retina, as irreversible damage to sensory retina develops only after 90-100 minutes of complete CRAO. Vision is 20/400 or worse in 66% of cases. | |||
Vision loss is permanent due to infarct of inner retina | |||
= References = | = References = | ||
<references /> | |||
Latest revision as of 15:14, March 18, 2025
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Cherry red spot is a significant fundoscopic finding in the macula, observed in central retinal artery occlusion (CRAO) and a variety of lipid storage disorders. The emphasis on this page is on CRAO, for which the presence of cherry red spot portends worse visual prognosis.
Disease Entity
Disease
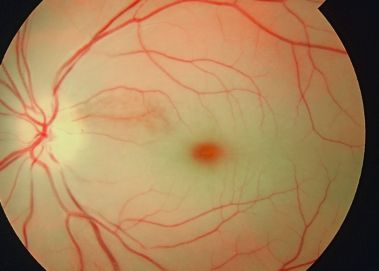
Cherry red spot at the macula is a clinically significant sign observed on fundus examination in a variety of pathological conditions[2], including retinal infarction, retinal ischemia, and several lysosomal storage disorders.[3] The term refers to the appearance of a red-tinted region at the center of the macula surrounded by retinal opacification[3], usually present due to the thickening and loss of transparency of the posterior pole of the retina.[4] The fovea retains relative transparency as it is the thinnest part of the retina and devoid of ganglion cells. The fovea receives its blood supply from the choroid, which is supplied by the long and short posterior ciliary arteries.[5] The appearance of a cherry red spot results when retinal edema, most commonly due to central retinal artery occlusion or traumatic retinal ischemia, causes the perimacular tissue of the retina to become less transparent, while the fovea maintains its normal color.[6][4] In the case of lipid storage diseases, the lipids are stored in the ganglion cell layer of the retina, giving the retina a white appearance. As ganglion cells are absent at the foveola, this area retains relative transparency, in contrast to the surrounding retina.[4] Cherry red spot is an important fundoscopic sign, and when found with key clinical features and a good history, often guides one to the diagnosis of the disease.[2] It should be noted that as a condition resolves or evolves over the course of time, the appearance of a cherry red spot diminishes and may not be present; therefore, absence of a cherry red spot does not necessarily indicate absence of disease.[3]
History
The term “cherry red spot” was first introduced in 1887 by American neurologist Bernard Sachs, who described the fundus appearance of a child with "amaurotic familial idiocy" in his publication on “arrested development with special reference to its cortical pathology”. [6] The patient was confirmed upon neuropathologic examination to have lipid storage in the brain. [7] Bernard Sachs acknowledged that his patient had also been examined by ophthalmologist Herman Joseph Knapp, who practiced in both New York and Berlin, and described the striking retinal findings at an ophthalmology meeting in Heidelberg using the term “cherry red color” to describe the fovea.[6] Knapp initially believed that the cherry red spot was a benign fundoscopic finding, but later realized its grave implications. [6]
Etiology
The causes of cherry red spot in the macula can be categorized by etiology:
Vascular
- Central Retinal Artery Occlusion (CRAO) - This disease entity characteristically presents as a sudden onset of painless, unilateral vision loss in an elderly patient. Cherry red spot has been found to be present on the initial examination in 90% of permanent CRAO cases and prognosticates poorer visual recovery. [3] CRAO is classified as arteritic and non-arteritic, which will further guide the management discussed below.
CRAO is most commonly (95% of cases) the non-arteritic CRAO, which is caused by atherothrombosis, thromboembolism, or vasospasm which occludes the central retinal artery. [3] Thrombus or thromboembolism may be composed of cholesterol, fibrin-platelet, or calcium; they usually originate from the carotid plaque and less commonly from the heart or the aorta. Associations with thromboembolic causes of CRAO include myxoma or vegetations of the cardiac valves and hypercoagulable states (hyperhomocysteinemia, factor V Leiden, protein C and S deficiencies, anti-thrombin III deficiencies, anti-phospholipid antibodies or prothrombin gene mutations, sickle cell disease). Non-thromboembolic vascular causes of CRAO include nocturnal arterial hypotension, retinal migraine, and transient vasospasm. [8][9] Arteritic CRAO occurs in the setting of inflammatory conditions like giant cell arteritis (GCA) and are an estimated 5% of CRAO cases. Other inflammatory disorders are less common causes of arteritic CRAO and include Susac syndrome, systemic lupus erythematosus, polyarteritis nodosa, and granulomatosis with polyangiitis.[10]
- Acute occlusion of the retinal circulation following cataract surgery (phacoemulsification) causing outer retinal whitening. [11]
- Compromise of the choroidal and retinal circulation due to intraorbital hemorrhage or mass. (Note that an ophthalmic artery occlusion may not manifest a cherry red spot due to compromise of both the choroidal and retinal circulations.)
- Macular infarction due to various causes such as retinal artery and vein occlusions, carotid artery occlusions, malignant hypertension, diabetes, radiation retinopathy, posterior uveitis, systemic inflammatory vasculopathies, and trauma. [12] [13][14][15]
Metabolic Storage Diseases
- GM 2 gangliosidosis – a group of autosomal-recessive disorders with deficiencies in lysosomal enzymes beta-hexosaminidase (HEX), including Tay-Sachs disease (beta hexoaminidase A deficiency) and Sandhoff (beta hexoaminidase A+B). New evidence suggests different disease cohorts with some presenting with early onset and rapid disease progression and others with later symptom onset and variable disease progression. There is an abnormal accumulation of gangliosides in the brain and ganglion cell layer of the retina. A cherry red spot is present in approximately 75-90% of the patients affected with early-onset disease. [17]
- Niemann-Pick disease - Type A, B, C, D disease
- GM1 gangliosidosis type 1 (Generalized gangliosidosis or Landing disease) - an autosomal recessive disorder caused by mutations in GLB1 in which a cherry-red spot in the macula occurs in around 50% cases
- Sialidosis or mucolipidosis type 1 - a lysosomal storage disease caused by alpha-neuraminidase deficiency (NEU1 gene mutation) which presents with ataxia, movement disorders, nystagmus, and myoclonic seizures. Intellect is usually normal, and somatic features are generally not seen. Two types have been described according to the age of onset and severity of the disease: Sialidosis type I or cherry red spot myoclonus syndrome is characterized by onset in the 2nd or 3rd decade, gait disturbances, myoclonus, ataxia, tremor, a cherry red spot at the macula, and seizures. The intellect and life expectancy are usually unaffected, though the patient eventually needs wheelchair assistance with time. Almost all the patients have a cherry red spot at the macula.
- Farber disease (Farber lipogranulomatosis) - a rare autosomal recessive (AR) disorder characterized by Ceramidase or acid ceramidase (chromosome 8p22) deficiency, affecting the GLA gene . The typical triad consists of painful swollen joints, subcutaneous nodules (lipogranulomas), and weak cry or hoarse voice due to laryngeal nodules. Other features include a developmental delay, visceromegaly, respiratory involvement, and neurological features, including quadriplegia, seizures, and myoclonus.
- Metachromatic leukodystrophy - another rare (AR) lysosomal storage disorder described by arylsulfatase A deficiency, resulting in abnormal collection of sulfatides in myelin-producing cells that leads to progressive destruction of white matter (leukodystrophy). A cherry red spot at the macula is occasionally found, along with progressive decline in intellectual and motor functions, peripheral neuropathy resulting in diminished sensations, hearing loss, incontinence, loss of speech and mobility, blindness, unresponsiveness, and eventual death.
- Galactosialidosis (Goldberg Cotlier syndrome or neuraminidase deficiency with beta-galactosidase deficiency or cathepsin A deficiency) is an AR disorder due to a mutation in the CTSA gene coding for cathepsin A. Ocular features include a cherry red spot at the macula, corneal clouding, and conjunctival telangiectasia.
Inflammation
- Retinitis involving the central retina, including progressive outer retinal necrosis and subacute sclerosing panencephalitis. [3]
Drug Toxicity
- Quinine
- Carbon monoxide
- Dapsone poisoning associated with hemolytic anemia and peripheral neuropathy
- Methanol
- Intravitreal gentamicin
Trauma
- Commotio Retinae - Acute retinal opacification following blunt trauma, also known as Berlin's edema, can cause a pseudo-cherry red spot, as a result of peripheral whitening of the retina that involves the macula.
- Optic Nerve Avulsion [18]
- Saturday Night Retinopathy - Occlusion of the retinal or ophthalmic artery due to headrest in a prolonged prone body position which results in self-induced sustained direct orbit compression and collapse of orbital vessels. A cherry red spot may appear if circulation in the choroid remains present.[19][20]
- Leukemic infiltrates of the optic nerve head may cause CRAO and cherry red spot
Risk Factors
Risk factors for cherry red spot due to non-arteritic CRAO are similar to those for acute ischemic stroke: diabetes, arterial hypertension, transient ischemic attack, coronary artery disease, smoking, and vascular disease. For retinal artery occlusion in particular, internal carotid artery stenosis is commonly associated, with a prevalence as high as 70% reported among patients with CRAO or branch retinal artery occlusion.[21]
Please refer to above section on etiologies for common associations with arteritic CRAO or other causes of cherry red spot.
Pathophysiology
The macula is histologically characterized by an area consisting of more than one layer of ganglion cell layers, so the ganglion cell layer is thicker at the macula. In disease entities that cause transparency loss (whitening or opacification) of the inner retina, the reddish color of the vascular choroid and pigmentation of retinal pigment epithelium (RPE) is not visible through the opacified retina. However, the foveola is devoid of the inner retinal layer. The retinal layers present at the foveola are (from vitreal to the sclera)- internal limiting membrane, outer nuclear layer, external limiting membrane, photoreceptor layer, and RPE. Inner retinal layers are absent in mature foveola. The vascular supply of the fovea is from the choroid and is not affected by occlusion of retinal vessels. Thus, the foveola, the thinnest part of the central retina, does not lose its transparency during inner retinal ischemia.
Thus, when there is inner retinal opacification, the reddish color of the vascular choroid and the RPE remain visible through the foveola, which is surrounded by an area of the white/opacified retina, giving rise to the typical cherry red spot. As described above, causes of opacification of the inner layers of the retina include compound deposition, as in storage diseases, and ischemia from vascular compromise involving the retinal but not the underlying choroidal circulation.
The size of the cherry red spot depends on the size of the foveola. As the color of the choroid and RPE varies with race, the color of the foveola too may vary by race. In Caucasian individuals, the foveola appears red, whereas in non-Caucasian patients; the color of foveola may vary and has been reported to be brown in a Canadian aboriginal child with Sandhoff disease and black in a patient of East Indian descent with Sandhoff disease. Thus, the alternate term ‘perifoveal white patch’ has been suggested. Note that when the ophthalmic artery is occluded, compromise to the vascular supply of the retina, choroid, and optic nerve head circulation leaves retinal opacification without a cherry red spot and the patient experiences severe vision loss with loss of light perception.
Opacification of the inner retina can occur as a result of retinal ischemia due to occlusion of the central retinal artery. The retinal whitening is typically most prominent in the posterior pole, and the opaque retina obscures the details of the choroidal vessels. The whitening of the retina becomes less prominent a little peripheral to the arcades, which may be related to the reduced inner retinal thickness at the peripheral retina. There is a cessation of axoplasmic transport and then the opacification of both the ganglion cell and the nerve fiber layer in a CRAO.
A variety of lipid storage disorders can produce deposition / accumulation of material at the level of the ganglion cell layer can other lipid storage disorders (sphingolipidoses), leading to progressive abnormal excessive intracellular accumulation of various glycolipids/phospholipids in the ganglion cell layer, that cause opacification of the inner retina. The foveola is devoid of the ganglion cell layer and hence remains transparent and allows transmission of the color of the choroid and the RPE. However, with increasing age, the damaged ganglion cells are lost, and the cherry red spot becomes less prominent. In such cases, there is consecutive optic atrophy and pallor of the optic disc due to the degeneration of the ganglion cell layer and nerve fiber layer.
Primary Prevention
Prevention for retinal ischemia in non-metabolic causes of cherry red spot include measures to reduce cardiovascular and cerebrovascular co-morbidities.
Diagnosis
Ruling out serious life-threatening or sight-threatening conditions is paramount in a patient who presents with a cherry red spot at the macula. Thorough evaluation is warranted, including attention to blood pressure.[3] Especially in the absence of clinical suspicion for alternative etiologies of cherry red spot (metabolic disease, recent surgery or trauma, drug use – see history below), acute loss of vision with a cherry red spot should raise concern for stroke since CRAO is an ocular variant of acute ischemic stroke. Hence, depending on institution, a stroke or neurology team may need to be consulted.
History
- Preceding history of amaurosis fugax (transient vision loss lasting seconds to minutes, but that may last up to 2 hours)
- Medical history of known cardiovascular risk factors (obesity, hypertension, diabetes, hypercholesterolemia), prior stroke or cerebrovascular disease, atrial fibrillation, or inherited or acquired thromboembolic states such as hyperhomocystenemia, factor V Leiden, protein C and S and anti-thrombin deficiencies, anti-phospholipid antibodies or prothrombin gene mutations, sickle cell disease, or neoplastic disease
- Known family or personal history of systemic inflammatory or autoimmune disease
- Tobacco use
- Family history of lipid/lysosomal storage disorders or metabolic disease
- Ocular trauma
- Face-down surgical procedures
- Drug use
Signs
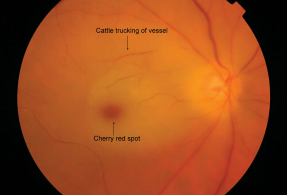
Fundoscopic features that present along with cherry red spot at the macula include:
- Yellow-white opacified appearance of the retina in the posterior pole, due to ischemic necrosis affecting the inner half of the retina, which vanishes in 4-6 weeks.
- Cloudy swelling of the retina
- Attenuated retinal arteries
- Thinning, dilation or normal appearance of retinal veins
- Slow segmental blood flow, known as “boxcarring” (“cattle trucking”) of the retinal arteries in severe obstruction.[9]
- Pallor of the optic disc (39% of CRAO cases) in later stages, as well as visible absence of nerve fiber layer in the region of optic disc[23]
- Presence of emboli (20-40% of CRAO cases)
- Pigmentary changes indicate that choroidal circulation is also involved.[23]
- Presence of a Hollenhorst plaque, which is a clinical finding in cases of central retinal artery occlusion, or one of its branches (BRAO) caused by a lodging embolus deriving from a cholesterol plaque. [8]
Additional ophthalmic signs concerning for acute CRAO include:
- Ipsilateral relative afferent pupillary defect
- Variable degrees of visual loss
- Impaired color vision proportional to impairment in visual acuity
Symptoms
- Sudden onset of painless monocular vision loss (visual acuity and/or peripheral vision) occurring over several seconds. Degree of visual loss may be variable.
- Symptoms of giant cell arteritis in older patients: new-onset headache, jaw claudication, scalp or posterior neck tenderness, proximal muscle and joint aches, anorexia, weight loss, fever
- Transient migraine due to vasospasm
- Neurologic symptoms indicative of lysosomal storage disorders or metabolic disease.
Clinical Diagnosis
- Spectral-Domain Optical Coherent Tomography (OCT) has been proposed as one modality that might be used to diagnose and monitor CRAO. In CRAO, there is an observed increase in intensity of inner retinal layers compared with age-matched controls, corresponding to the layers supplied by central retinal arteries.[24] OCT can be correlated with visual prognosis.[24] Incomplete CRAO shows minimal retinal architectural disruption and inner layer hyper-reflectivity without retinal edema. Subtotal CRAO demonstrates inner macular thickening and loss of organization of the inner retina, and total CRAO demonstrates marked inner retinal thickening and subfoveal choroidal thinning. [25][26] In the chronic phase, there is a corresponding thinning of the inner retinal layers.
- Fluorescein Angiography may be a prognostic test in CRAO, as poor perfusion on fluorescein angiography has been associated with lower vision than exudative and mixed perfusion. However, this finding does not influence therapy.[27] Normal choroidal filling begins 1-2 seconds before retinal filling and is complete within 5 seconds of dye appearance in healthy eyes.[27]A delay of 5 or more seconds is seen in 10% of patients.[27] Ophthalmic artery occlusion or carotid artery obstruction should be considered if choroidal filling is significantly delayed. A delay is noted in arteriovenous transit time (reference range, < 11 seconds), as well as a delay in retinal arterial filling. Arterial narrowing with normal fluorescein transit is observed after recanalization.[27]
- Optical Coherent Tomography Angiography provides structural and functional (blood flow) information at a fixed point but is not useful to appreciate leakage from vessels.[28] OCTA shows decreased vascular perfusion in superficial and deep retinal plexus that corresponds to poor perfusion on fluorescein angiography.[29] However, unlike fundus fluorescein angiography, OCTA cannot demonstrate a delay in transit time. [30]
- Fundus Autofluorescence: in the acute phase of CRAO, fundus autofluorescence in ischemic areas is decreased because of retinal edema blocking the normal RPE. Eventually, this could return to normal baseline or may be associated with increased autofluorescence owing to a window defect created by the thinned-out inner retinal layers. [28]
- Electroretinography shows a diminished b-wave corresponding to Muller and/or bipolar cell ischemia.
Diagnostic procedures
- Blood pressure and pulse monitoring, particularly to detect atrial fibrillation, possibly with Holter monitor
- Electrocardiogram (ECG) to look for arrhythmia (in particular atrial fibrillation) and other cardiac disease
- Echocardiography if there is a specific indication such as a history of rheumatoid fever or known cardiac valvular disease.
- Chest x-ray to investigate for sarcoidosis, tuberculosis, left ventricular hypertrophy in hypertension
- Magnetic Resonance Imaging (MRI), as approximately 20% of patients with a CRAO also have cerebral ischemia; therefore a brain MRI may reveal concurrent cerebral ischemia in patients without accompanying neurological symptoms.[31]
- Magnetic Resonance Angiography (MRA) of the head and neck may be more accurate in detecting vascular occlusive disease.
- Computerized tomography (CT) for rapid stroke evaluation or Computerized Tomography Angiography (CT/CTA) or MRI/MRA of the neck if suspecting carotid disease or dissection
- Carotid Doppler Ultrasonography to evaluate for atherosclerotic plaque and arterial flow if suspecting occlusive carotid disease
Laboratory test
- Complete blood count CBC (anemia, leukemia, polycythemia, platelet disorders)
- Basic metabolic panel to check urea and electrolytes
- Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP) to screen for giant cell arteritis in elderly patients
- Hypercoagulablity work-up (e.g, factor V Leiden, prothrombin mutation, homocysteine levels, fibrinogen, anti-phospholipid antibodies, prothrombin time/activated partial thromboplastin time [PT/aPTT]) if suspicious for coagulopathy
- Plasma protein electrophoresis if suspicious for sickle cell disease
- Fasting blood sugar, cholesterol, triglycerides, and lipid panel to evaluate risk factors for atherosclerotic disease
- Blood cultures if suspecting bacterial endocarditis and septic emboli
- Antibodies for suspected vasculitidies or rheumatologic disease
- Genetic testing, molecular analysis of cells or tissues, and enzyme assays if considering lipid-storage disorders in younger patients
Differential diagnosis
A macular hemorrhage or hole, with resulting enhanced red contrast relative to the surrounding retina, may be confused with a cherry red spot.[6]
Other causes of acute painless loss of vision include vitreous and chorioretinal hemorrhage, retinal detachment, and acute optic neuropathy.
Management
Cherry red spot at the macula is a clinically significant fundoscopic finding that usually denotes an ocular emergency, especially in the patient who presents with sudden, painless unilateral vision loss and absence of other preceding clinical factors. The general management of a cherry red spot sign is determined by the underlying pathological condition that causes the appearance of the fundoscopic finding.
In many institutions, suspicion of CRAO, a form of acute ischemic stroke, warrants urgent neurology consultation for stroke work-up.[32]In addition to appropriate stroke work-up and fundoscopic exam, the patient should be screened for arteritis if in the appropriate demographic.
For metabolic storage disorders, management requires a team of various specialties. Generalized pearls for the management of such patients include avoiding/reducing intake of specific molecules that lead to accumulation within ganglion cells, enzyme replacement therapy, and symptomatic management.
General treatment
Treatment may vary depending on etiology but given the relative frequency and clinical significance of CRAO, discussion here will focus on treatment of CRAO. Please also refer to the page on Retinal Artery Occlusion for further details.
In the acute setting of CRAO, treatment options are directed at resolving the CRAO and circulation, and thus maximizing visual outcome. Experimental studies suggest no detectable optic nerve or retinal damage in primate models with CRAO if retinal blood flow is restored within 90 minutes. Subsequent partial recovery may be possible if ischemia is reversed within 240 minutes. However, occlusions lasting longer than 240 minutes produce irreversible damage.[33][34] The treatment approach is attempted within the first 24 hours.
For various storage diseases, the management requires a team of various specialties. Generalized pearls for the management of such patients include avoiding/reducing intake of specific molecules that lead to accumulation within ganglion cells, enzyme replacement therapy, and symptomatic management.
Medical therapy
IOP reducing pharmacological agents
- IV acetazolamide
- IV mannitol
- Topical antiglaucoma medications
Vasodilation agents
- Pentoxifylline
- Inhalation of carbogen
- Sublingual isosorbide dinitrate
Thrombolytic therapy to dissolve clot
- IV or intra-arterial recombinant tissue plasminogen activator (rt-PA) is delivered through the catheterization of the ophthalmic artery or supraorbital artery and can reach the CRA in doses 100 times greater than by systemic administration. Vision improvement is noted in 50% of the patients.[10]
IV methylprednisolone
Hyperbaric oxygen therapy
Procedures / Surgery
- Ocular massage: an attempt to mechanically dislodge the obstructing embolus using in and out movement with the use of a three-mirror (Goldmann) contact lens or digital massage. Pressure should be applied for 10-15 seconds followed by sudden release. Ocular massage can cause arterial dilatation and improve retinal perfusion by an 86% increase in flow volume.[10]
- Anterior chamber paracentesis: provides sudden decrease in intraocular pressure, and aids the perfusion pressure behind the obstruction to dislocate the obstructing embolus.[10]
- Transluminal Nd:YAG laser embolysis: this procedure has been advocated for CRAO or BRAO, in cases when the occluding embolus is visible. Shots of 0.5–1.0 mJ or higher are applied directly to the embolus using a fundus contact lens, in order to lyse or dislodge the occluding embolus. [10] Embolectomy has been said to occur if the embolus is ejected into the vitreous via a hole in the arteriole. The main complication is vitreous hemorrhage.
- Pars plana vitrectomy: Surgical removal of the clot
Complications
Neovascularization is a complication that may occur in patients with CRAO with an incidence that varies from 3.0-18.8%. It may involve the retina, iris, or iridocorneal angle. Moreover, the detection of neovascularization following CRAO has ranged from as early as the day of presentation to 2 years after the CRAO diagnosis. The association of CRAO and the development of neovascular glaucoma is thought to occur in about 5%; however, a prospective study established a temporal relationship between CRAO and neovascular glaucoma in 15% of cases. These results are corroborated by studies supporting the occurrence around 8 weeks from diagnosis to clinically evident neovascularization.[9] However, a prospective study of 232 eyes found neovascularization in 2.5% of cases, and the authors found that there was no causal relationship.[35]
With frequent visits, many cases of neovascularization can be managed early with treatments such as panretinal photocoagulation to decrease retinal oxygen demand and off-label intravitreal bevacizumab. Since neovascularization can occur early, regular follow-up appointments should be required, especially within the first 4 months.[35]
Prognosis
The initial management approach described above may provide some benefit; however, there is no clear evidence at this point that these measures result in improvement of the patient's final visual acuity and have the best chance when performed shortly after the diagnosis of CRAO. Vision loss is permanent due to infarct of inner retina, as irreversible damage to sensory retina develops only after 90-100 minutes of complete CRAO. Vision is 20/400 or worse in 66% of cases.
References
- ↑ https://commons.wikimedia.org/wiki/File:Cherry_red_spot_in_patient_with_central_retinal_artery_occlusion_(CRAO).jpg
- ↑ Jump up to: 2.0 2.1 Suvarna J C, Hajela S A. Cherry-red spot. J Postgrad Med 2008;54:54-7
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Tripathy K, Patel BC. Cherry Red Spot. [Updated 2021 Feb 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539841/
- ↑ Jump up to: 4.0 4.1 4.2 General Practice Notebook
- ↑ USMLE First AID 2010 page 417
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Leavitt, J. A., & Kotagal, S. (2007). The “Cherry Red” Spot. Pediatric Neurology, 37(1), 74-75 doi:10.1016/j.pediatrneurol.2007.04.011
- ↑ Leavitt, J. A., & Kotagal, S. (2007). The “Cherry Red” Spot. Pediatric Neurology, 37(1), 74–75. doi:10.1016/j.pediatrneurol.2007.04.011
- ↑ Jump up to: 8.0 8.1 Kaufman EJ, Mahabadi N, Patel BC. Hollenhorst Plaque. [Updated 2021 Feb 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470445/
- ↑ Jump up to: 9.0 9.1 9.2 Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013 Jun;27(6):688-97. doi: 10.1038/eye.2013.25. Epub 2013 Mar 8. PMID: 23470793; PMCID: PMC3682348.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 Sim, S., & Ting, D. (2017, August). Diagnosis and Management of Central Retinal Artery Occlusion. American Academy of Ophthalmology. https://www.aao.org/eyenet/article/diagnosis-and-management-of-crao.
- ↑ Central retinal artery occlusion after phacoemulsification under peribulbar anaesthesia: S Rodríguez Villa,R Salazar Méndez,M Cubillas Martín,M Cuesta García. Eur J Ophthalmol. 2021 Mar;31(2):NP77 PMID: 26652970 DOI: 10.1016/j.oftal.2015.10.003
- ↑ Gass J.D.M. A fluorescein angiographic study of macular dysfunction secondary to retinal vascular disease. Arch Ophthalmol. 1968;80:535–617.
- ↑ Bentley C.R., Stanford M.R., Shilling J.S., Sanders M.D., Graham E.M. Macular ischaemia in posterior uveitis. Eye (Lond) 1993;7:411–414.
- ↑ Acacio I., Goldberg M.F. Peripapillary and macular vessel occlusions in sickle cell anemia. Am J Ophthalmol. 1973;75:861–866.
- ↑ Goel N, Rajput M, Sawhney A, Sardana T. Macular infarction and traumatic optic neuropathy following blunt ocular trauma. Saudi J Ophthalmol. 2016;30(1):53-55. doi:10.1016/j.sjopt.2015.08.002
- ↑ Learning About Tay-Sachs Disease [Internet]. 2011 [cited 2013 Oct 20] Available from: http://www.genome.gov/10001220
- ↑ Kern J, Böhringer J, Timmann D, et al.. Clinical, Imaging, Genetic, and Disease Course Characteristics in Patients With GM2 Gangliosidosis. Neurology. 2024; 102 (1): doi: 10.1212/WNL.0000000000207898
- ↑ Sudden occlusion of the retinal and posterior choroidal circulations in a youth. Brown GC, Magargal LE.Brown GC, et al. Am J Ophthalmol. 1979 Oct;88(4):690-3. doi: 10.1016/0002-9394(79)90666-4.
- ↑ Self-induced Orbital Compression Injury: Saturday Night Retinopathy. Williams AL, Greven M, Houston SK, Mehta S.Williams AL, et al. JAMA Ophthalmol. 2015 Aug;133(8):963-5. doi: 10.1001/jamaophthalmol.2015.1114.
- ↑ Williams AL, Greven M, Houston SK, Mehta S. Self-induced Orbital Compression Injury: Saturday Night Retinopathy. JAMA Ophthalmol. 2015;133(8):963–965. doi:10.1001/jamaophthalmol.2015.1114
- ↑ Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002;33:1963–1967. doi: 10.1161/01.str.0000023445.20454.a8
- ↑ Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013 Jun;27(6):688-97. doi: 10.1038/eye.2013.25. Epub 2013 Mar 8. PMID: 23470793; PMCID: PMC3682348
- ↑ Jump up to: 23.0 23.1 Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina. 2007 Mar;27(3):276-89. doi: 10.1097/01.iae.0000238095.97104.9b. PMID: 17460582.
- ↑ Jump up to: 24.0 24.1 Chen H, Chen X, Qiu Z, Xiang D, Chen W, Shi F, et al. Quantitative analysis of retinal layers' optical intensities on 3D optical coherence tomography for central retinal artery occlusion. Sci Rep. 2015 Mar 18. 5:9269.
- ↑ Chen H, Chen X, Qiu Z, Xiang D, Chen W, Shi F, et al. Quantitative analysis of retinal layers' optical intensities on 3D optical coherence tomography for central retinal artery occlusion. Sci Rep. 2015 Mar 18. 5:9269.
- ↑ Mehta N, Marco RD, Goldhardt R, Modi Y. Central Retinal Artery Occlusion: Acute Management and Treatment. Curr Ophthalmol Rep. 2017 Jun. 5 (2):149-159.
- ↑ Jump up to: 27.0 27.1 27.2 27.3 Gong H, Song Q, Wang L. Manifestations of central retinal artery occlusion revealed by fundus fluorescein angiography are associated with the degree of visual loss. Exp Ther Med. 2016 Jun. 11 (6):2420-2424.
- ↑ Jump up to: 28.0 28.1 Mathew R, Papavasileiou E, Sivaprasad S. Autofluorescence and high-definition optical coherence tomography of retinal artery occlusions. Clin Ophthalmol. 2010 Oct 21. 4:1159-63.
- ↑ de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015. 1:5.
- ↑ Bonini Filho MA, Adhi M, de Carlo TE, Ferrara D, Baumal CR, Witkin AJ, et al. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN RETINAL ARTERY OCCLUSION. Retina. 2015 Nov. 35 (11):2339-46.
- ↑ Dattilo M, Biousse V, Newman NJ. Update on the Management of Central Retinal Artery Occlusion. Neurol Clin. 2017 Feb. 35 (1):83-100.
- ↑ Terao R, Fujino R, Ahmed T. Risk Factors and Treatment Strategy for Retinal Vascular Occlusive Diseases. J Clin Med. 2022 Oct 27;11(21):6340. doi: 10.3390/jcm11216340. PMID: 36362567; PMCID: PMC9656338.
- ↑ Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion: retinal survival time. Exp Eye Res. 2004;78:723–736. doi: 10.1016 s0014-4835(03)00214-8
- ↑ Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol. 2000;129:786–795. doi: 10.1016/s0002-9394(00)00384-6
- ↑ Jump up to: 35.0 35.1 Jung YH, Ahn SJ, Hong JH, et al. Incidence and Clinical Features of Neovascularization of the Iris following Acute Central Retinal Artery Occlusion. Korean J Ophthalmol. 2016;30(5):352-359. doi:10.3341/kjo.2016.30.5.352