Vogt’s Striae
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Vogt’s Striae
Disease
Vogt’s striae are vertical lines in the posterior stroma of Descemet’s membrane of the cornea. They disappear with pressure applied to the cornea.[1] Vogt’s striae have been reported to be horizontal in only two cases in the literature.[2] Vogt’s striae are a feature of keratoconus, but they are also found in healthy eyes.[1][3]
Etiology
Vogt’s Striae are found in 65% of patients with keratoconus.[4] They have also been seen in 82% of eyes in patients with and without keratoconus.[3]
Risk Factors
Eye rubbing can lead to keratoconus, which has an increased number of striae.[3][5][6]
General Pathology
The cornea and sclera make up the outer surface of the eyeball and protect the eye. The cornea is transparent and contributes 70% of the refracting power in the eye. The cornea consists of epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium.[7] Striae can be attributed to undulations, not ruptures, in continuous collagen lamellae of the stroma. Lamellae are 1-3 µm thick. The undulations originate at Descemet’s membrane and travel through the corneal stroma toward Bowman’s layer. Some striae may reach Bowman’s layer, while some end in the mid or anterior stroma.[3]
The stroma is made up of collagen I, VI, and XII, and makes up 80-85% of the thickness of the cornea. The thickness of the cornea is proportional to changes in the amount of collagen. The cornea is thinnest in the center and thickens toward the periphery.[7]
Striae decrease in number and width with age as the cornea stiffens. Striae have been observed in healthy patients, patients with keratoconus, and patients with corneal edema and opacities. Striae may show “criss-crossing orientation”. Stromal striae consist of collagen IV, laminin, and the keratan-sulfate-containing proteoglycans keratocan and lumican.[3]
Striae can be seen in 82% of eyes. It has been postulated that striae are protective of collagen fibrils against external mechanical forces and increased intraocular pressure (IOP) as they disappear with pressure. IOP increases with physiological eye rubbing. Striae present as 6 striae/mm, with a width of 20 µm, and length of 42% of the stromal thickness on average.[3]
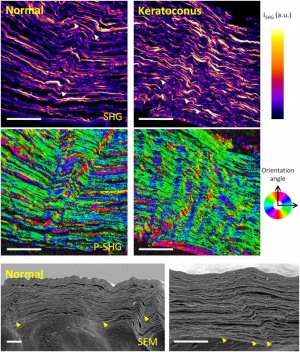
Striae in the normal cornea have been described as few, short, and oblique; striae in the keratoconus cornea are numerous and vertical. Striae in the edematous cornea (Fuch’s dystrophy and bullous keratopathy) are few and oblique (Fig. 1, 2).[3]


It had been previously postulated that striae were due to ruptures in collagen lamellae, but it has been demonstrated by second harmonic generation microscopy (SHG), polarization-resolved SHG (P-SHG), and scanning electron microscopy (SEM) that striae are due to folds in collagen lamellae (Fig. 3).[3]
Pathophysiology
Keratoconus[11]
Keratoconus is a noninflammatory condition in which the cornea becomes conical in shape due to thinning of corneal stroma.[11][12] The changes in the cornea lead to irregular astigmatism, myopia, and protrusion. Vision can decrease dramatically but does not lead total blindness. Keratoconus affects both eyes but may present initially unilaterally. Signs of keratoconus include stromal thinning, conical protrusion, Fleischer’s ring (an iron line surrounding the cone) and stromal striae (Vogt’s striae).[12]
Keratoconus (KCN) is bilateral, but was documented once in the literature unilaterally in a patient with admitted eye rubbing of the affected eye.[6] Keratoconus has been associated with patients with atopy who frequently rub their eyes due to irritation.[5] The theorized cause of keratoconus due to physical rubbing is mechanical fatigue of the cornea.[6] Striae may protect collagen fibrils against increased extraocular pressure, such as from eye rubbing. Stromal striae affect corneal viscoelasticity. Viscoelasticity of the cornea allows for maintenance of corneal shape and dissipation of pressure energy. In keratoconus, striae increase in number and become vertical. This corresponds to changes in corneal viscoelasticity. Keratoconus eyes have decreased viscoelasticity, and striae are primarily found in the keratoconus zone of the cornea. Striae are a risk factor for Descemet’s membrane rupture in keratoconus (hydrops).[3]
Keratoconus eyes with Vogt’s striae have deeper anterior chamber depth and thinner corneas than keratoconus eyes without Vogt’s striae.[13] KCN eyes have worse visual acuity, refractive errors, and corneal tomographic parameters when Vogt’s striae are present.[13] Vogt’s striae may be a cause of biomechanical deterioration in keratoconus eyes. Vogt’s striae are typically “parallel to the steep axis of the KCN cone.”[14] There is a difference in intraocular pressure between eyes with and without Vogt’s striae . Eyes with Vogt’s striae have a mean IOP of 13.76 ± 1.16 mmHg, while eyes without Vogt’s striae have a mean IOP of 14.15 ± 1.28 mmHg.[14] Vogt’s striae make corneas weaker in KCN eyes. KCN eyes with Vogt’s striae have lower corneal hysteresis (CH), corneal resistance factor (CRH), and Goldman correlated IOP but greater difference between CH and CRF (CH-CRF) than eyes without Vogt’s striae. Higher CH-CRF indicates worse corneal biomechanics. KCN eyes with Vogt’s striae had significantly higher uncorrected and corrected distance visual acuity, maximum keratometry, mean keratometry, and Jackson’s cross cylinder with axes at 0 and 90 degrees (J0). There was no significant difference in Jackson’s cross cylinder with axes at 45 and 135 degrees (J45) between the two groups of eyes. KCN eyes with Vogt’s striae had lower radius, central corneal thickness, and IOP, but greater deformation amplitude and peak distance.[14]
Primary prevention
Striae are a naturally appearing phenomenon in healthy and diseased eyes.[3]
Diagnosis
Striae can be diagnosed with slit-lamp, optical coherence tomography (OCT), full-field optical coherence microscopy (FFOCM), and confocal microscopy (CM). OCT has the least sensitivity in detecting striae as the width of the striae is at the lateral resolution limit for OCT. Striae appear as dark bands on CM, FFOCM, and OCT because the undulated lamellae reflect light away.[3]

Differential diagnosis
Vogt’s striae are similar in appearance but must be differentiated from Descemet’s membrane folds. Descemet’s membrane is the basement membrane of the corneal endothelium, made of collagen type IV and laminin.[16] Descemet’s membrane folds can result after surgery to treat keratoconus, such as deep anterior lamellar keratoplasty. Descemet’s membrane folds tend to improve with time and do not have a lasting impact on vision, except in rare cases. In the case in which Descemet’s folds persisted, one patient experienced ghosting, blurring, glare, and starbursts.[17] Descemet’s folds can also occur due to a pterygium.[16]
Management
Treat the underlying keratoconus to prevent progression of Vogt’s striae, as KCN eyes with Vogt’s striae have worse biomechanics than KCN eyes without Vogt’s striae.[13] In healthy eyes, striae may protect against increased IOP, which can be cause by eye rubbing, and do not require treatment.[3]
References
- ↑ 1.0 1.1 Mocan, M. C., Yilmaz, P. T., Irkec, M. and Orhan, M. (2008), The significance of Vogt's striae in keratoconus as evaluated by in vivo confocal microscopy. Clin Exp Ophthalmol, 36: 329-334.
- ↑ Güngör IU, Beden U, Sönmez B. Bilateral horizontal Vogt's striae in keratoconus. Clin Ophthalmol. 2008;2(3):653–655.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Grieve K, Ghoubay D, Georgeon C, Latour G, Nahas A, Plamann K, Crotti C, Bocheux R, Borderie M, Nguyen T, Andreiuolo F, Schanne-Klein M, and Borderie V. Stromal striae: a new insight into corneal physiology and mechanics. Sci Rep. 2017;7(1):13584
- ↑ Zadnik K, Barr JT, Edrington TB, et al. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study. Invest Ophthalmol Vis Sci. 1998;39(13):2537–2546
- ↑ 5.0 5.1 Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84(8):834–836
- ↑ 6.0 6.1 6.2 Bral N, Termote K. Unilateral Keratoconus after Chronic Eye Rubbing by the Nondominant Hand. Case Rep Ophthalmol. 2017;8(3):558–561
- ↑ 7.0 7.1 Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190–194.
- ↑ Grieve K, Ghoubay D, Georgeon C, Latour G, Nahas A, Plamann K, Crotti C, Bocheux R, Borderie M, Nguyen T, Andreiuolo F, Schanne-Klein M, and Borderie V. Stromal striae: a new insight into corneal physiology and mechanics. Sci Rep. 2017;7(1):13584
- ↑ Grieve K, Ghoubay D, Georgeon C, Latour G, Nahas A, Plamann K, Crotti C, Bocheux R, Borderie M, Nguyen T, Andreiuolo F, Schanne-Klein M, and Borderie V. Stromal striae: a new insight into corneal physiology and mechanics. Sci Rep. 2017;7(1):13584
- ↑ Grieve K, Ghoubay D, Georgeon C, Latour G, Nahas A, Plamann K, Crotti C, Bocheux R, Borderie M, Nguyen T, Andreiuolo F, Schanne-Klein M, and Borderie V. Stromal striae: a new insight into corneal physiology and mechanics. Sci Rep. 2017;7(1):13584
- ↑ 11.0 11.1 Kozak A, Feldman BH, Sheth S, Rose L, Asbell PA, Plumb RC. Keratoconus. EyeWiki. https://eyewiki.aao.org/Keratoconus. Accessed August 2, 2019.
- ↑ 12.0 12.1 Rabinowitz YS, Keratoconus RYS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319.
- ↑ 13.0 13.1 13.2 Ostadi-Moghaddam H, Sedaghat MR, Rakhshandadi T, Rajabi S, Narooie-Noori F, Askarizadeh F. A contralateral eye study comparing characteristics of corneal endothelial cells in bilateral keratoconus patients with unilateral corneal Vogt's striae. J Curr Ophthalmol. 2018;30(3):228–233.
- ↑ 14.0 14.1 14.2 Askarizadeh F, Sedaghat MR, Ostadi-Moghaddam H, Narooie-Noori F, Rakhshandadi T, Rajabi S. A Contralateral Eye Study Comparing Corneal Biomechanics in Subjects with Bilateral Keratoconus with Unilateral Vogt's Striae. Med Hypothesis Discov Innov Ophthalmol. 2017;6(2):49–55
- ↑ Khaled ML, Helwa I, Drewry M, Seremwe M, Estes A, Liu Y. Molecular and Histopathological Changes Associated with Keratoconus. Biomed Res Int 2017;2017:7803029
- ↑ 16.0 16.1 Solomon R, Ehrenhaus M, Palmer C, et al. Gaze-induced Descemet's folds secondary to a primary pterygium. Eye Contact Lens. 2005;31(6):288-290.
- ↑ Mohamed SRH, Manna A, Amissah-Arthur K, McDonnell PJ. Non-resolving Descemet folds 2 years following deep anterior lamellar keratoplasty: The impact on visual outcome. Contact Lens & Anterior Eye, 2009;32(6):300-302.

