Visual Variant of Alzheimer’s Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
The visual variant of Alzheimer’s disease (VVAD), also known as Posterior Cortical Atrophy (PCA) or Benson’s syndrome, is a rare neurodegenerative disease. It is characterized by progressive higher order visual dysfunction with relative sparing of other cognitive functions until late in the course. The majority of cases share pathological features with Alzheimer’s Disease (AD).
VVAD typically may present in the mid-50s (earlier than classic AD), and is an under-recognized cause of the visual disturbance. There are generally no abnormalities on routine ophthalmic or neurological examination. Symptoms are of visuospatial or visuoperceptual dysfunction, such as difficulty with reading, navigating, numeracy, and interpretation of complex visual scenes. Diagnosis is based on the clinical syndrome with radiological evidence of posterior brain atrophy. Management is largely supportive and will not slow progression; however, early multidisciplinary intervention can maintain functional independence and improve quality of life.
Disease Entity
Disease
VVAD is not specifically recognized in the International Classification of Diseases (ICD) version 10 nomenclature:
ICD-10
G31.1 Senile degeneration of the brain, not elsewhere classified
History
The term ‘posterior cortical atrophy’ was first introduced by Benson and colleagues in a 1988 report.[1] Benson presented five dementia patients noteworthy for early disturbance of higher order visual function with relative preservation of memory and insight. These patients had predominant parieto-occipital atrophy on neuroimaging, and later developed symptoms of Balint’s and Gerstmann’s syndromes.
Balint’s syndrome was first described in 1909 by Rezsö Bálint, and similarities with PCA have been noted by several investigators.[2] Interestingly, a constellation of symptoms akin to PCA was also reported by Arnold Pick in 1902, the psychiatrist best known for another lobar-specific disease (Pick’s frontotemporal dementia).[3]
Definitions
VVAD may be an early-onset, progressive neurodegenerative disease with predominant visual dysfunction and atrophy of posterior cortical regions.[4] Although most cases share a pathological basis with AD, the underlying etiology is heterogeneous, and so the condition is defined by the clinical-radiological syndrome. Numerous clinical diagnostic criteria have been proposed[5][6][7] such as that of Mendez and colleagues (Table 1).[8] An international working party has recently been formed to develop consensus diagnostic criteria, aiming to advance research into diagnosis, etiology, and treatment.[4]
| Table 1. Proposed diagnostic criteria for posterior cortical atrophy |
|---|
1 Core diagnostic features (all must be present)
|
2 Supportive diagnostic features
|
Epidemiology
Poor general awareness coupled with the lack of formal diagnostic criteria have led to underdiagnosis of VVAD in the past, and a paucity of epidemiological studies mean that prevalence and incidence remain uncertain. In a study of patients at a neurological dementia clinic, 5% (24 of 523) presented with predominant visual symptoms consistent with PCA.[9]
VVAD tends to affect a younger demographic than AD, with age of onset typically 50-65 years.[6][8][10] Some studies have reported a slight female predominance[5][11] though others have found no gender difference.[8][12]
Classification
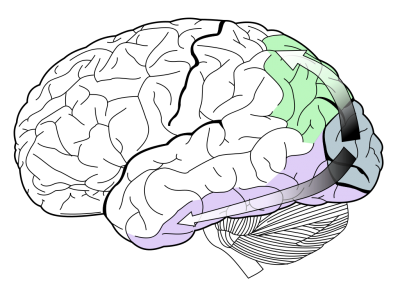
Two major subtypes of PCA have been proposed, ‘dorsal’ (occipito-parietal) and ‘ventral’ (occipito-temporal), based on which cortical visual stream is predominantly affected by neurodegeneration (Figure 1).[13][14][15][16][17] The dorsal (‘where’) stream is thought to have principally visuospatial complaints, with difficulties in visually guided motor movements and object location manifesting as apraxia, agraphia, and eventually complete Balint’s syndrome. The ventral (‘what’) stream is believed to suffer mostly visuoperceptive dysfunction, with impaired recognition of objects (visual agnosia), faces (prosopagnosia), and writing (agraphia).
This classification has been disputed by some investigators, who suggest the subtypes could be better described as a continuum within PCA rather than distinct phenotypes.[10][11] The initial subtyping was based on extrapolation of PCA case reports to the ‘two streams’ model of visual processing.[16][18] Contemporary case series have found limited evidence of distinct pathological streams, with significant overlap on neuropsychological testing and neuroimaging between behaviourally defined streams.[6][11]
Pathophysiology
Although the vast majority are due to Alzheimer’s disease pathology, the pathological basis of PCA is heterogeneous. Other neuropathologies implicated include corticobasal degeneration,[5][12][19] Lewy body dementia,[5][12] prion disease (including Creutzfeldt-Jacob disease and fatal familial insomnia),[12][20] and subcortical gliosis.[20] At least 80% of PCA cases are due to AD pathology, and have increased density of neurofibrillary tangles and amyloid plaques at autopsy.[5][12][21][22][23] PCA and AD patients also show analogous changes on cerebrospinal fluid biomarkers, including total tau, phosphorylated tau, and beta amyloid (Aβ).[24][25][26][27] Both demonstrate high cortical binding of amyloid on positron emission tomography (PET).[28]
The specific distribution of pathological changes in VVAD compared to AD remains uncertain. Some investigators have found differences in the location of plaques between the two entities,[22][21][25] while others have reported similar distribution.[5][12][25][29]
Genetics
The exact genetic basis of VVAD remains elusive, and is likely heterogeneous. Mutations have been reported in the presenilin 1 and 2 genes (PSEN1 and PSEN 2),[30] prion protein gene (PRNP),[31] IT15,[32] microtubule-associated protein tau gene (MAPT),[33] and progranulin gene (GRN).[14] The limited available data found no significant difference in PCA risk in those with a positive family history of dementia.[5][8]
Genetic studies are ongoing to further define the nosology of degenerative dementias.[34] A large genome-wide assocation study of 302 PCA patients recently identified three novel loci (SEMA3C, CNTNAP5, and FAM46A) as potential genes of interest.
Differentiating from Alzheimer’s disease
VVAD has a different presentation and course than that of typical Alzheimer’s, though the two phenotypes may converge late in the disease.[35] VVAD patients tend to be younger and have complex visual dysfunction, with sparing of other cognitive functions in early disease. In particular, VVAD is not associated with the episodic memory deficits characteristic of classic AD, and patients retain good insight into their deterioration. Although AD may be associated with visual agnosias and ocular motor dysfunction (ocular apraxia, delayed saccades, and impaired smooth pursuit), these are secondary to the memory and functional complaints. Neuropsychological testing will generally not exhibit the visuospatial and visuoperceptual dysfunction seen in VVAD. Neuroimaging demonstrates greater atrophy in posterior (parieto-occipital) brain regions, with relative sparing of memory centres in the medial temporal lobe.[7][11][36] Both the clinical picture and imaging findings often converge in later disease, with VVAD progressing to global cognitive dysfunction.[4][37]
Diagnosis
The clinical picture of VVAD is of complex visuoperceptual and visuospatial impairments, typically with normal ophthalmic findings. Characteristic features are summarised in Table 2. As noted earlier, consensus diagnostic criteria have not yet been established.[4]
| Table 2. Characteristic features of posterior cortical atrophy | |||
|---|---|---|---|
Core features
| |||
Supportive features
|
History
The clinical presentation of VVAD is variable, affected by the individual pattern of neuropsychological impairment and time to specialist referral. Given the preservation of insight, memory, and language skills, anxiety and depression are common presenting features.[10] Earliest reported symptoms are non-specific, including visual blurring and glare sensitivity. Agnosias, oculomotor apraxia, and positive perceptual phenomena combine to give difficulties with complex visual behaviours such as reading and driving.[10] Difficulty reading is due to oculomotor apraxia (generating saccades to track word to word), visual disorientation (difficulty following lines of text), visual crowding (identifying letters when neighbouring stimuli are present), and reverse-size phenomenon (easier reading of small than large print).[1][10][38][39][40][41] Patients may also report troubles with reading an analogue clock face, depth perception, and perception of patterns or textured surfaces.[10][42] As the disorder progresses, patients may describe prosopagnosia (misrecognition of familiar faces) and topographical or environmental disorientation (getting lost in familiar places). Some have difficulty with numerical calculation (acalculia), spelling, or making coordinated movements (dyspraxia).[10][42] Unusual visual manifestations are also reported, such as contrast sensitivity,[43] prolonged colour after-images,[44] and difficulty identifying static objects within the visual field.[41][42][45]
Physical examination
Ophthalmic examination in most cases of VVAD is unremarkable. Occasionally, higher order dysfunction may falsely impair visual acuity and visual field testing. Neurological examination may reveal extrapyramidal signs (in approximately 41% of PCA patients), myoclonus (in 24%), palmar grasp reflex (in 26%),[9] or limb apraxias.[7] Visual hallucinations may also be noted (in approximately 25%).[5][6][46]
Neuropsychological features
Specific neuropsychological examination is required to elicit the salient features of PCA; that is, visuospatial and visuoperceptual dysfunction.[1][5][8][12][35][47][48] These include:
- Alexia
- Visual agnosias, including topographical (environmental) agnosia, prosopagnosia
- Balint’s syndrome: simultanagnosia, optic ataxia, and oculomotor apraxia
- Gerstmann’s syndrome: agraphia, acalculia, right-left confusion, and finger agnosia
A complete Balint’s or Gerstmann’s syndrome is rare at diagnosis.[5][6][48] A recent study by Singh and colleagues found that the most common clinical features in VVAD were simultanagnosia (in 92% of patients), agraphia (68%), poly-mini-myoclonus (64%) and oculomotor apraxia (56.5%).[49]
Simultanagnosia is a disorder in which patients can recognise individual elements of a complex visual scene but are unable to grasp the overall meaning.[48][50] This restricted window of visual attention can be thought of as ‘seeing the trees but not the forest’.[42][51] Simultanagnosia appears to be the most prevalent and therefore sensitive feature in early PCA (affecting approximately 85%).[49] It is caused by lesions in the region of the parieto-occipital junction, especially Brodmann area 7 and the superior occipital cortex.[50][52] No quantitative tests for simultanagnosia are available, however a useful bedside screening tool is pseudoisochromatic plates (eg Ishihara). Colour vision is unaffected, however the overall figure cannot be synthesised from the multiple smaller elements.[53] A similar test can be performed with Navon figures (Figure 2).[51] Patients may also be asked to interpret a complex visual scene, such as the commonly used “Boston Cookie Theft” picture.
Optic ataxia refers to a lack of coordination of visually guided hand movements (eg reaching, grasping).[54][55] It is caused by lesions of the intraparietal sulcus.[56] To test for optic ataxia, the patient should fixate on the clinician’s nose, and attempt to reach for the clinician’s finger presented in the peripheral visual field.[42][48][55]
Oculomotor apraxia is an inability to voluntarily guide eye movements, referred to by Bálint as “psychic paralysis of gaze”.[2] Patients have difficulty initiating saccades to a particular visual target, and impaired visual scanning.[48] Oculomotor abnormalities are almost ubiquitous in PCA.[57] This feature is caused by damage to the posterior parietal cortices.[42] To test for oculomotor apraxia, the patient should saccade between two visual targets. Optokinetic testing will also show absence of fast phase nystagmus, and patients will demonstrate impaired smooth pursuit.[58]
The verbal and performance intelligence quotients (IQ) of the Wechsler Adult Intelligence Scale are also valuable tools. Patients tend to demonstrate significantly lower scores on performance compared to verbal testing.[10]
Clinical diagnosis
There is often delayed diagnosis of PCA given the young age at onset, unusual symptomatology, and unremarkable standard ophthalmic examination. Co-existing mood disorders contribute to misdiagnosis as a ‘functional’ or non-organic disorder. These may even prompt unnecessary procedures such as cataract surgery.[45][47][59][60]
Presence of visual dysfunction without evidence of ocular abnormalities should prompt consideration of neuroimaging and referral to a neurologist. Simple office-based testing of reading, writing, simple arithmetic, pseudoisochromatic plates, Navon figures, and interpretation of complex scenes will aid in diagnosis.
Ancillary tests
Visual field defects are frequently present, however likely underdiagnosed because of the difficulties in accurate perimetry assessment.[42] Homonymous field defects including hemianopias or quadrantanopia are most common.[5][10][45][61] These deficits may be variable and poorly reproducible secondary to the cognitive dysfunction, potentially contributing to misdiagnosis as a non-organic pathology. Field defects occasionally occur in classic AD, most commonly of the bilateral inferior fields.[60]
Neuroimaging
As the term suggests, PCA is associated with atrophy predominantly affecting the occipitoparietal and occipitotemporal cortices.[8][11][17][49][62] Automated studies using cross-sectional voxel-based morphometry and cortical thickness have demonstrated greater right parietal and less left medial temporal and hippocampal atrophy in patients with PCA compared to typical AD.[11][36][62] There is, however, considerable overlap in the patterns of atrophy between VVAD and AD, with similar regions affected including the posterior cingulate, precuneus, and inferior parietal lobe.[11][36] This supports the hypothesis that VVAD is a phenotypic variant of AD pathology. Longitudinal analysis demonstrates correlation of structural atrophy on imaging with the progression to a more global dementia state.[37]
Functional neuroimaging reflects the pattern seen on structural imaging.[63] Single photon emission computed tomography (SPECT) and fluorodeoxyglucose (FDG)-PET imaging demonstrate hypoperfusion and hypometabolism in occipitoparietal regions.[7][64][65] FDG-PET has also revealed hypometabolism in the frontal eye fields bilaterally, thought to be secondary to a loss of input from the occipitoparietal regions and potentially the mechanism behind oculomotor apraxia.[7][63]
Novel imaging modalities also support the pathologic basis of VVAD. Pittsburgh compound B (PiB)-PET imaging demonstrates increased accumulation of beta amyloid, with some studies demonstrating increased amyloid deposition in the occipitoparietal region relative to classic AD.[26][66][67] Other reports found no significant difference in amyloid deposition between the two.[25][28] There is emerging evidence that tau imaging may show a stronger association with AD variants than amyloid imaging.[68]
Laboratory tests
There is no definitive laboratory test for VVAD. Formal neuropsychological testing, bloods, and neuroimaging are required to exclude treatable causes such as neoplastic, infective, or inflammatory diseases. CSF biomarkers may provide evidence of Alzheimer’s pathology, but there are no specific markers for the PCA syndrome.
Differential diagnosis
The rarity and unusual symptomatology of VVAD can often lead to misdiagnosis. Differential diagnosis includes:
- Classic Alzheimer’s. Clinically distinct (as discussed earlier), with more pronounced memory and functional deficits, and sparing of the mesial temporal lobes on neuroimaging
- Lewy Body Dementia. Consider as the primary complaint with prominent visual hallucinations and rapid eye movement (REM) sleep behaviour disorder[46]
- Intracerebral space occupying lesion (eg tumour, abscess) or infection (meningoencephalitis)
- Creutzfeldt-Jakob disease (CJD). May present with primary visual complaints, but progression is more rapid than VVAD
- Progressive multifocal leukoencephalopathy. Can present with isolated occipital features in immunocompromised patients
- Migraine. May account for discrete episodes of glare sensitivity and visuoperceptual disturbances
- Occipital lobe seizures
- Primary mood disorders (anxiety, depression). May be considered once PCA has been excluded as an underlying cause
Management
No cure for VVAD exists at this time and there is limited evidence for specific treatment paradigms. However, pharmacological and non-pharmacological therapies have the potential to improve daily functioning and quality of life. Vision therapy may be recommended.
Medical therapy
Given the underlying pathology, acetylcholinesterase inhibitors approved for classic AD are frequently used in VVAD.[10][42][45] These medications including donepezil (marketed as Aricept), galantamine (Reminyl), and rivastigmine (Exelon) are thought to provide some symptomatic relief in PCA patients with AD or Lewy Body pathology. Efficacy is uncertain, and evidence is limited to expert recommendation and case reports.[10][69]
Consideration must also be given to treatment of co-existing conditions. Patients with features of Parkinsonism could be trialled on antiparkinson medications. Anxiolytic or antidepressant medications may benefit those with prominent mood symptoms, a common concern in individuals who preserve insight into their functional decline.
Non-pharmacological therapy
Timely diagnosis of VVAD is essential for reassurance and early intervention to maintain functional capacity. Psychoeducation for the patient and family regarding visual difficulties and expected prognosis is important. Early referral to a vision therapy or low-vision center may be of assistance. Patients often benefit from visual aids and resources such as voice recognition software, devices with simplified displays, cooking and hygiene aids, and appropriate lighting.[70] A useful and comprehensive list of home safety recommendations has been prepared (see https://www.raredementiasupport.org/posterior-cortical-atrophy/living-with-pca/). Driving is not advisable for the majority of VVAD patients. Publically funded financial assistance or support services for low vision may be accessible after review by an ophthalmologist.[10]
Functional and home assessments by an occupational therapist and physiotherapist can significantly improve quality of life. The aim is to encourage development of compensatory strategies to maintain independence in activities of daily living for as long as possible. Structured multidisciplinary rehabilitation programs including psychoeducation, functional aids, cognitive training, and compensatory strategies have shown some benefits.[71][72][73][74] There is scant evidence for the efficacy of any particular approach.
Referral should be made to appropriate VVAD specific support resources, such as the National Institute on Aging’s Alzheimer’s Disease Education and Referral Centre (ADEAR) (https://www.nia.nih.gov/alzheimers) and the Alzheimer’s Association (http://www.alz.org).
Disease progression
VVAD typically progresses to a global cognitive dysfunction resembling that of classic AD. Episodic memory loss, linguistic and executive dysfunction may gradually develop.[5][21][37] Patients are thought to have similar life expectancy to that of typical Alzheimer’s disease, though evidence is lacking and others may live longer.
References
- ↑ Jump up to: 1.0 1.1 1.2 Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45(7):789-793.
- ↑ Jump up to: 2.0 2.1 Bálint R. Die seelenlahmung des ‘Schauens’. Monatschr Psychiatr Neurol. 1909;1:25-51.
- ↑ Pick A. Über eine eigenthümliche Sehstörung senil Dementer. Jahrb Psychiatr Neurol. 1902;22:35-44.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Crutch SJ, Schott JM, Rabinovici GD, et al. Shining a light on posterior cortical atrophy. Alzheimers Dement. 2013;9(4):463-465.
- ↑ Jump up to: 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168-1174.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 McMonagle P, Deering F, Berliner Y, Kertesz A. The cognitive profile of posterior cortical atrophy. Neurology. 2006;66(3):331-338.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 Kas A, de Souza LC, Samri D, et al. Neural correlates of cognitive impairment in posterior cortical atrophy. Brain. 2011;134(Pt 5):1464-1478.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14(1):33-40.
- ↑ Jump up to: 9.0 9.1 Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007;43(7):835-845.
- ↑ Jump up to: 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012;11(2):170-178.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Lehmann M, Crutch SJ, Ridgway GR, et al. Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer's disease. Neurobiol Aging. 2011;32(8):1466-1476.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Renner JA, Burns JM, Hou CE, McKeel DW, Jr., Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175-1180.
- ↑ Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130(Pt 10):2636-2645.
- ↑ Jump up to: 14.0 14.1 Caroppo P, Belin C, Grabli D, et al. Posterior cortical atrophy as an extreme phenotype of GRN mutations. JAMA Neurol. 2015;72(2):224-228.
- ↑ Goldstein MA, Ivanov I, Silverman ME. Posterior cortical atrophy: an exemplar for renovating diagnostic formulation in neuropsychiatry. Compr Psychiatry. 2011;52(3):326-333.
- ↑ Jump up to: 16.0 16.1 Ross SJ, Graham N, Stuart-Green L, et al. Progressive biparietal atrophy: an atypical presentation of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1996;61(4):388-395.
- ↑ Jump up to: 17.0 17.1 Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123 Pt 3:484-498.
- ↑ Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15(1):20-25.
- ↑ Jellinger KA, Grazer A, Petrovic K, et al. Four-repeat tauopathy clinically presenting as posterior cortical atrophy: atypical corticobasal degeneration? Acta Neuropathol. 2011;121(2):267-277.
- ↑ Jump up to: 20.0 20.1 Victoroff J, Ross GW, Benson DF, Verity MA, Vinters HV. Posterior cortical atrophy. Neuropathologic correlations. Arch Neurol. 1994;51(3):269-274.
- ↑ Jump up to: 21.0 21.1 21.2 Levine DN, Lee JM, Fisher CM. The visual variant of Alzheimer's disease: a clinicopathologic case study. Neurology. 1993;43(2):305-313.
- ↑ Jump up to: 22.0 22.1 Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer's disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res. 1997;37(24):3609-3625.
- ↑ Bokde AL, Pietrini P, Ibanez V, et al. The effect of brain atrophy on cerebral hypometabolism in the visual variant of Alzheimer disease. Arch Neurol. 2001;58(3):480-486.
- ↑ Seguin J, Formaglio M, Perret-Liaudet A, et al. CSF biomarkers in posterior cortical atrophy. Neurology. 2011;76(21):1782-1788.
- ↑ Jump up to: 25.0 25.1 25.2 25.3 Rosenbloom MH, Alkalay A, Agarwal N, et al. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76(21):1789-1796.
- ↑ Jump up to: 26.0 26.1 Formaglio M, Costes N, Seguin J, et al. In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings. J Neurol. 2011;258(10):1841-1851.
- ↑ de Souza LC, Lamari F, Belliard S, et al. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer's disease from other cortical dementias. J Neurol Neurosurg Psychiatry. 2011;82(3):240-246.
- ↑ Jump up to: 28.0 28.1 de Souza LC, Corlier F, Habert MO, et al. Similar amyloid-beta burden in posterior cortical atrophy and Alzheimer's disease. Brain. 2011;134(Pt 7):2036-2043.
- ↑ Lehmann M, Ghosh PM, Madison C, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain. 2013;136(Pt 3):844-858.
- ↑ Sitek EJ, Narozanska E, Peplonska B, et al. A patient with posterior cortical atrophy possesses a novel mutation in the presenilin 1 gene. PLoS One. 2013;8(4):e61074.
- ↑ Depaz R, Haik S, Peoc'h K, et al. Long-standing prion dementia manifesting as posterior cortical atrophy. Alzheimer Dis Assoc Disord. 2012;26(3):289-292.
- ↑ Caixeta L. Huntington's disease presenting as posterior cortical atrophy. Arq Neuropsiquiatr. 2011;69(2b):407-408.
- ↑ Rossi G, Bastone A, Piccoli E, et al. Different mutations at V363 MAPT codon are associated with atypical clinical phenotypes and show unusual structural and functional features. Neurobiol Aging. 2014;35(2):408-417.
- ↑ Peng G, Liu P, He F, Luo B. Posterior cortical atrophy as a primary clinical phenotype of corticobasal syndrome with a progranulin gene rs5848 TT genotype. Orphanet J Rare Dis. 2016;11:13.
- ↑ Jump up to: 35.0 35.1 Charles RF, Hillis AE. Posterior cortical atrophy: clinical presentation and cognitive deficits compared to Alzheimer's disease. Behav Neurol. 2005;16(1):15-23.
- ↑ Jump up to: 36.0 36.1 36.2 Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73(19):1571-1578.
- ↑ Jump up to: 37.0 37.1 37.2 Lehmann M, Barnes J, Ridgway GR, et al. Global gray matter changes in posterior cortical atrophy: a serial imaging study. Alzheimers Dement. 2012;8(6):502-512.
- ↑ Stark ME, Grafman J, Fertig E. A restricted 'spotlight' of attention in visual object recognition. Neuropsychologia. 1997;35(9):1233-1249.
- ↑ Mendez MF, Cherrier MM. The evolution of alexia and simultanagnosia in posterior cortical atrophy. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(2):76-82.
- ↑ Ardila A, Rosselli M, Arvizu L, Kuljis RO. Alexia and agraphia in posterior cortical atrophy. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10(1):52-59.
- ↑ Jump up to: 41.0 41.1 Crutch SJ, Lehmann M, Gorgoraptis N, et al. Abnormal visual phenomena in posterior cortical atrophy. Neurocase. 2011;17(2):160-177.
- ↑ Jump up to: 42.0 42.1 42.2 42.3 42.4 42.5 42.6 42.7 Beh SC, Muthusamy B, Calabresi P, et al. Hiding in plain sight: a closer look at posterior cortical atrophy. Pract Neurol. 2015;15(1):5-13.
- ↑ Cronin-Golomb A, Sugiura R, Corkin S, Growdon JH. Incomplete achromatopsia in Alzheimer's disease. Neurobiol Aging. 1993;14(5):471-477.
- ↑ Chan D, Crutch SJ, Warrington EK. A disorder of colour perception associated with abnormal colour after-images: a defect of the primary visual cortex. J Neurol Neurosurg Psychiatry. 2001;71(4):515-517.
- ↑ Jump up to: 45.0 45.1 45.2 45.3 Lee AG, Martin CO. Neuro-ophthalmic findings in the visual variant of Alzheimer's disease. Ophthalmology. 2004;111(2):376-380; discussion 380-371.
- ↑ Jump up to: 46.0 46.1 Josephs KA, Whitwell JL, Boeve BF, et al. Visual hallucinations in posterior cortical atrophy. Arch Neurol. 2006;63(10):1427-1432.
- ↑ Jump up to: 47.0 47.1 Giannakopoulos P, Gold G, Duc M, Michel JP, Hof PR, Bouras C. Neuroanatomic correlates of visual agnosia in Alzheimer's disease: a clinicopathologic study. Neurology. 1999;52(1):71-77.
- ↑ Jump up to: 48.0 48.1 48.2 48.3 48.4 Rizzo M, Vecera SP. Psychoanatomical substrates of Balint's syndrome. J Neurol Neurosurg Psychiatry. 2002;72(2):162-178.
- ↑ Jump up to: 49.0 49.1 49.2 Singh TD, Josephs KA, Machulda MM, et al. Clinical, FDG and amyloid PET imaging in posterior cortical atrophy. J Neurol. 2015;262(6):1483-1492.
- ↑ Jump up to: 50.0 50.1 Coslett HB, Saffran E. Simultanagnosia. To see but not two see. Brain. 1991;114 ( Pt 4):1523-1545.
- ↑ Jump up to: 51.0 51.1 Navon D. Forest before trees: The precedence of global features in visual perception. Cognit Psychol. 1977;9:353-383.
- ↑ Dalrymple KA, Barton JJ, Kingstone A. A world unglued: simultanagnosia as a spatial restriction of attention. Front Hum Neurosci. 2013;7:145.
- ↑ Brazis PW, Graff-Radford NR, Newman NJ, Lee AG. Ishihara color plates as a test for simultanagnosia. Am J Ophthalmol. 1998;126(6):850-851.
- ↑ Perenin MT, Vighetto A. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain. 1988;111 ( Pt 3):643-674.
- ↑ Jump up to: 55.0 55.1 Meek BP, Shelton P, Marotta JJ. Posterior cortical atrophy: visuomotor deficits in reaching and grasping. Front Hum Neurosci. 2013;7:294.
- ↑ Battaglia-Mayer A, Caminiti R. Optic ataxia as a result of the breakdown of the global tuning fields of parietal neurones. Brain. 2002;125(Pt 2):225-237.
- ↑ Shakespeare TJ, Kaski D, Yong KX, et al. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain. 2015;138(Pt 7):1976-1991.
- ↑ Le Ber I, Brice A, Durr A. New autosomal recessive cerebellar ataxias with oculomotor apraxia. Curr Neurol Neurosci Rep. 2005;5(5):411-417.
- ↑ Zakzanis KK, Boulos MI. Posterior cortical atrophy. Neurologist. 2001;7(6):341-349.
- ↑ Jump up to: 60.0 60.1 Trick GL, Trick LR, Morris P, Wolf M. Visual field loss in senile dementia of the Alzheimer's type. Neurology. 1995;45(1):68-74.
- ↑ Rene R, Munoz S, Campdelacreu J, et al. Complex visual manifestations of posterior cortical atrophy. J Neuroophthalmol. 2012;32(4):307-312.
- ↑ Jump up to: 62.0 62.1 Whitwell JL, Jack CR, Jr., Kantarci K, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2007;28(7):1051-1061.
- ↑ Jump up to: 63.0 63.1 Nestor PJ, Caine D, Fryer TD, Clarke J, Hodges JR. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer's disease) with FDG-PET. J Neurol Neurosurg Psychiatry. 2003;74(11):1521-1529.
- ↑ Gardini S, Concari L, Pagliara S, Ghetti C, Venneri A, Caffarra P. Visuo-spatial imagery impairment in posterior cortical atrophy: a cognitive and SPECT study. Behav Neurol. 2011;24(2):123-132.
- ↑ Pietrini P, Furey ML, Graff-Radford N, et al. Preferential metabolic involvement of visual cortical areas in a subtype of Alzheimer's disease: clinical implications. Am J Psychiatry. 1996;153(10):1261-1268.
- ↑ Ng SY, Villemagne VL, Masters CL, Rowe CC. Evaluating atypical dementia syndromes using positron emission tomography with carbon 11 labeled Pittsburgh Compound B. Arch Neurol. 2007;64(8):1140-1144.
- ↑ Tenovuo O, Kemppainen N, Aalto S, Nagren K, Rinne JO. Posterior cortical atrophy: a rare form of dementia with in vivo evidence of amyloid-beta accumulation. J Alzheimers Dis. 2008;15(3):351-355.
- ↑ Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016.
- ↑ Kim E, Lee Y, Lee J, Han SH. A case with cholinesterase inhibitor responsive asymmetric posterior cortical atrophy. Clin Neurol Neurosurg. 2005;108(1):97-101.
- ↑ Yong KX, Rajdev K, Shakespeare TJ, Leff AP, Crutch SJ. Facilitating text reading in posterior cortical atrophy. Neurology. 2015;85(4):339-348.
- ↑ Shakespeare TJ, Yong KX, Foxe D, Hodges J, Crutch SJ. Pronounced impairment of everyday skills and self-care in posterior cortical atrophy. J Alzheimers Dis. 2015;43(2):381-384.
- ↑ Alves J, Magalhaes R, Arantes M, Cruz S, Goncalves OF, Sampaio A. Cognitive rehabilitation in a visual variant of Alzheimer's disease. Appl Neuropsychol Adult. 2015;22(1):73-78.
- ↑ Roca M, Gleichgerrcht E, Torralva T, Manes F. Cognitive rehabilitation in posterior cortical atrophy. Neuropsychol Rehabil. 2010;20(4):528-540.
- ↑ Weill-Chounlamountry A, Poncet F, Crop S, et al. Physical medicine and rehabilitation multidisciplinary approach in a case of posterior cortical atrophy. Ann Phys Rehabil Med. 2012;55(6):430-439.