Tonometry after Refractive Surgery
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Numerous studies have documented a change in measured intraocular pressure (IOP) after LASIK, PRK, and other refractive surgeries. While the procedures themselves may cause a change in intraocular pressure, as with Steroid Glaucoma after LASIK or interface fluid[1], in most cases the true IOP remains the same and only the measurement is affected. The change in corneal biomechanics after refractive surgery causes a false underestimation of IOP that is more pronounced with applanation and indentation tonometry. Since a falsely low IOP reading may lead to a delay in diagnosis of ocular hypertension or glaucoma, ophthalmologists should be aware of the difficulty in obtaining true IOP readings after refractive surgery.
Background
By reshaping the cornea to induce a more favorable refraction, refractive surgery alters the cornea’s biomechanical properties. These changes include a decrease in corneal hysteresis, flattening of the corneal curvature, and most importantly a decrease in the central corneal thickness (CCT)[2]. Any tonometry that relies on one of these properties will therefore yield different readings before and after surgery, even if the “true” IOP is unchanged.
Not all tonometric methods are significantly affected by refractive surgery, however. While a detailed discussion of the theory behind IOP and Tonometry is left for other articles, a brief summary of the major tonometric techniques and their utility after refractive surgery follows.
Applanation Tonometry
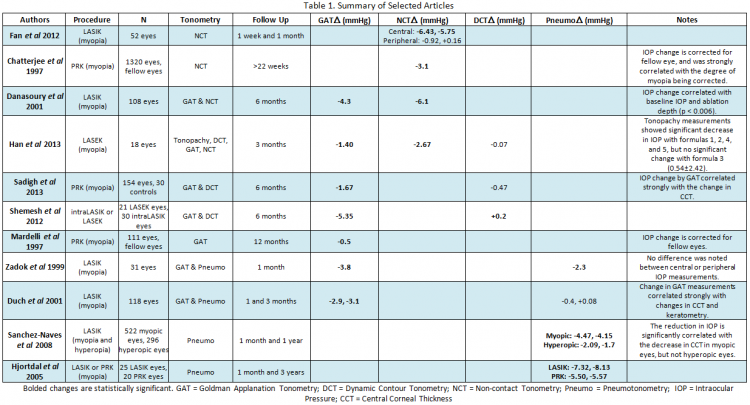
In applanation tonometry, the IOP is estimated from the force required to flatten a certain area of the cornea. Goldman Applanation Tonometry (GAT), the current gold standard, measures the force required to flatten a 3.06 mm area on the cornea by placing a prism directly on the cornea. Non-Contact Tonometry (NCT) or “air-puff” tonometry uses a similar principle, but with a gradually increasing column of air rather than a prism. These techniques assume that the eye is a uniform sphere, however, and were also developed with an “ideal” CCT of 520 micrometers. Since refractive surgery significantly changes both of these assumptions, a change in IOP by GAT and NCT after LASIK and PRK was quickly noticed (Table 1)[3][4][5]. Similarly significant changes in measured IOP have since been documented in LASEK[6].
As expected, the decrease in measured IOP by GAT has been found to be strongly correlated with the ablation depth, change in CCT, and baseline IOP. The change in IOP has been inconsistent from study to study, however, with some studies finding minimal change[4] and others reporting decreases in excess of 5 mmHg[7]. As a result, to date no corrective formulas have been established. While recent studies have suggested that the inaccuracy of applanation tonometry may be ameliorated by measuring the peripheral corneal IOP rather than a central IOP, thereby avoiding the area of greatest biomechanical change[7], other studies have not found any difference between central and peripheral measurements[8]. Due to the established inaccuracy of applanation tonometry after refractive surgery, GAT and NCT measurements should be interpreted with caution after refractive surgery.
Indentation Tonometry
In indentation tonometry, a known weight is used to press a certain depth into the cornea, with higher weights corresponding to higher IOP. Since the tonometer’s weight is opposed by corneal rigidity, which is in turn dependent on CCT, indentation tonometry measurements should be affected by refractive surgery. Of the available indentation tonometry methods, which include Schiotz tonometry and Tono-Pens, only contact pneumotonometers have been extensively studied after refractive surgery; unfortunately, pneumotonometers combine both applanation and indentation tonometry into one device, which limits conclusions about indentation tonometry specifically. As expected, most studies found a significant decrease in IOP measured by pneumotonometry after LASIK or PRK[8][9][10] that persisted for at least one year, although others found no significant change[11]. Peripheral measurements have not been found to increase the accuracy of pneumotonometry[8]. Further research is needed to elucidate the effectiveness of indentation tonometers after refractive surgery, but current research suggests that they fall prey to the same faults as applanation tonometry.
Dynamic Contour Tonometry
Dynamic contour tonometry (DCT) uses a piezoelectric sensor to dynamically detect changes in the intraocular pressure. The tonometer’s tip is designed to cradle the cornea without deforming the surface, which theoretically makes this technique less reliant on corneal thickness or hysteresis. Since the tip is shaped for an ideal cornea, flattening of the cornea during refractive surgery was once thought to affect DCT measurements, but this has not been borne out by studies. Multiple experiments on the use of DCT after LASIK, PRK, and LASEK have found that of all the tonometric methods, DCT is the least affected by refractive surgery[12][6][13] with virtually no significant change in measured IOP noted in the immediate or late post-operative periods. DCT is therefore the recommended technique for measurement of IOP after refractive surgery; however, limited office availability may restrict its routine use in post-refractive care.
Interface fluid syndrome after LASIK
It is important to have a high degree of suspicion for interface fluid syndrome in any patient with progressive glaucoma and a history of LASIK.[14][15][16][17]Interface fluid can lead to falsely low IOP readings with traditional tonometry. This can happen any anypoint after LASIK and there are case reports of late onset interface fluid syndrome, even 20 years after LASIK.[18] These patients need IOP checked in the periphery to ensure adequate evaluation of IOP.
Conclusion
After refractive surgery, intraocular pressure should be closely monitored for the complications of ocular hypertension or glaucoma. Due to the procedure-induced changes in corneal thickness, hysteresis, and curvature, however, the measurement of IOP may be falsely low if performed with applanation or indentation tonometry. The change in measured IOP is strongly correlated with the ablation depth and change in central corneal thickness, but as of yet, no corrective formulas have been established. Dynamic contour tonometry is the preferred method for measurement for IOP due to its minimal dependence on corneal characteristics, but may not be widely available. Ophthalmologists should be aware of the issues with IOP measurement after LASIK, PRK, and LASEK, and should closely monitor refractive surgery patients for glaucomatous changes despite a seemingly normal IOP.
References
- ↑ Hamilton DR, Manche EE, Rich LF, Maloney RK. Steroidinduced glaucoma after laser in situ keratomileusis associated with interface fluid. Ophthalmology 2002;109:659–65.
- ↑ Tsai AS, Loon SC. Intraocular pressure assessment after laser in situ keratomileusis: a review. Clinical and Experimental Ophthalmology 2012;40:295-304.
- ↑ Chatterjee A, Shah S, Bessant DA, Naroo SA, Doyle SJ. Reduction in Intraocular Pressure after Excimer Laser Photorefractive Keratectomy : Correlation with Pretreatment Myopia. Ophthalmology 1997;104:355-359.
- ↑ Jump up to: 4.0 4.1 Mardelli PG, Piebenga LW, Whitacre MM, Siegmund KD. The Effect of Excimer Laser Photorefractive Keratectomy on Intraocular Pressure Measurements Using the Goldmann Applanation Tonometer. Ophthalmology 1997;104:945-949.
- ↑ El Danasoury MA, El Maghraby A, Coorpender SJ. Change in intraocular pressure in myopic eyes measured with contact and non-contact tonometers after laser in situ keratomileusis. Journal of Refractive Surgery 2001;17:97-104.
- ↑ Jump up to: 6.0 6.1 Han KE, Kim H, Kim NR, Jun I, Kim EK, Kim TI. Comparison of intraocular pressures after myopic laser-assisted subepithelial keratectomy: tonometry-pachymetry, Goldmann applanation tonometry, dynamic contour tonometry, and noncontact tonometry. Journal of Cataract and Refractive Surgery 2013;39:888-897.
- ↑ Jump up to: 7.0 7.1 Fan Q, Zhang J, Zheng L, Feng H, Wang H. Intraocular pressure change after myopic laser in situ keratomileusis as measured on the central and peripheral cornea. Clinical and Experimental Ophthalmology 2012;95:421-426.
- ↑ Jump up to: 8.0 8.1 8.2 Zadok D, Tran DB, Twa M, Carpenter M, Schanzlin DJ. Pneumotonometry versus Goldmann tonometry after laser in situ keratomileusis for myopia. Journal of Cataract and Refractive Surgery 1999;25:1344-1348.
- ↑ Hjortdal JO, Moller-Pedersen T, Ivarsen A. Biomechanical Changes in the Human Cornea After PRK and LASIK. Investigative Ophthalmology and Visual Science 2005;46:2735-B288.
- ↑ Duch S, Serra A, Castanera J, Abos R, Quintana M. Tonometry after laser in situ keratomileusis treatment. Journal of Glaucoma 2001;10:261-265.
- ↑ Sadigh AL, Fouladi RJ, Hashemi H, Beheshtnejad AH. A comparison between Goldmann applanation tonometry and dynamic contour tonometry after photorefractive keratectomy. Graefe's archive for clinical and experimental ophthalmology 2013;251:603-608.
- ↑ Shemesh G, Soiberman U, Kurtz S. Intraocular pressure measurements with Goldmann applanation tonometry and dynamic contour tonometry in eyes after IntraLASIK or LASEK. Clinical Ophthalmology 2012;6:1967-1970.
- ↑ Mansoori T. Bilateral interface fluid syndrome and glaucoma progression after laser-assisted in situ keratomileusis. Oman J Ophthalmol. 2023 Mar 27;16(2):329-332. doi: 10.4103/ojo.ojo_112_22. PMID: 37602170; PMCID: PMC10433060.
- ↑ Shoji N, Ishida A, Haruki T, Matsumura K, Kasahara M, Shimizu K. Interface Fluid Syndrome Induced by Uncontrolled Intraocular Pressure Without Triggering Factors After LASIK in a Glaucoma Patient: A Case Report. Medicine (Baltimore). 2015 Sep;94(39):e1609. doi: 10.1097/MD.0000000000001609. PMID: 26426645; PMCID: PMC4616883.
- ↑ Lyle WA, Jin GJ, Jin Y. Interface fluid after laser in situ keratomileusis. J Refract Surg. 2003 Jul-Aug;19(4):455-9. doi: 10.3928/1081-597X-20030701-13. PMID: 12899478.
- ↑ Hamilton DR, Manche EE, Rich LF, Maloney RK. Steroid-induced glaucoma after laser in situ keratomileusis associated with interface fluid. Ophthalmology. 2002 Apr;109(4):659-65. doi: 10.1016/s0161-6420(01)01023-5. PMID: 11927421.
- ↑ Koronis S, Diafas A, Tzamalis A, Samouilidou M, Mataftsi A, Ziakas N. Late-onset interface fluid syndrome: A case report and literature review. Semin Ophthalmol. 2022 Oct-Nov;37(7-8):839-848. doi: 10.1080/08820538.2022.2102928. Epub 2022 Jul 21. PMID: 35866219.