Systemic Chemotherapy and Ocular Toxicities
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Targeted anti-cancer agents have dramatically improved survival for a variety of cancers. These drugs disrupt cellular signaling and development pathways that are vital for tumor cell growth and proliferation. However, treatment can be accompanied by an array of ocular adverse effects, the incidence of which varies by medication class. The toxicities range from mild effects that can be managed conservatively to severe complications that pose an immediate threat to vision.
Mitogen Activating Protein Kinase (MAPK) Pathway Inhibitors
MEK inhibitors mentioned in Drug Induced Maculopathy article.
Starting in 2014, MEK and BRAF inhibitors were FDA-approved for treatment of metastatic melanoma through blocking, either alone or in combination, several downstream kinases in the mitogen-activated protein kinase signaling pathway [1]. Specifically, mutations in the BRAF kinase lead to a constitutively active pathway resulting in tumor cell proliferation. Specifically, the BRAF V600E mutation is associated with the development in melanoma, Hodgkin’s lymphoma, hairy cell leukemia, lung adenocarcinoma, and colorectal carcinomas. Inhibition of the BRAF protein increases T-cell tumor infiltration and activity [2]. Similarly, inhibition of MEK unleashes an anti-tumor immune response and less VEGF release [3]. This is due to MEK inhibition no longer stimulating IGF-1 induced VEGF production [4]. Current BRAF inhibitors include vemurafenib, dabrafenib, and encorafenib, and current MEK inhibitors are trametinib, cobimetinib, and binimetinib.
Ocular treatment related adverse effects (TRAE) can occur with MEK or BRAF use or when used in combination. The most common ocular TRAE with BRAF inhibitor monotherapy is anterior or intermediate uveitis. MEK inhibitor monotherapy has associations with retinal vein occlusions, multifocal serous retinal detachments that are dose-dependent, and panuveitis [5][6]. Combination therapy is additive and can result in any of the above complications.
Management of MEK or BRAF associated ocular TRAEs includes observation versus use of topical or periocular corticosteroids [7]. MEK inhibitor-associated serous retinopathy, has been described to be self-limited and may subside after weeks or months with or without discontinuation of the MEK inhibitor [5]. Cases of uveitis associated with BRAF inhibitor use should again be a diagnosis of exclusion and can be treated with topical or local corticosteroids [7].
ERK inhibitors have also been associated with a similar retinopathy as seen in MEK inhibitors, with potentially more cystoid macular edema and intraretinal fluid [8][9]. A case series of 20 patients showed retinopathy was reversible and did not cause serious eye damage [8].
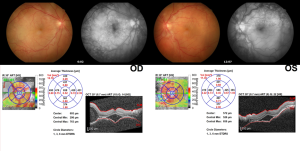
Antibody Drug Conjugates
Antibody drug conjugates mentioned here.
Immune Checkpoint Inhibitors
Antibody drug conjugates mentioned here.
Epidermal Growth Factor Receptor (EGFR) Inhibitors
Epidermal growth factor receptor (EGFR) is a tyrosine kinase family involved in cell signaling proliferation, and angiogenesis. Over-expression of EGFR can lead to neoplasia and tumor development and is associated with many malignancies including lung, head, neck, breast, colon, and brain cancers [10].
EGFR inhibitors used for treatment of non-small cell lung cancer include gefitinib, erlotinib, wafatinib, panitumumab, and cetuximab. Lapatinib and neratinib are EGFR inhibitors used for the treatment of HER2+ breast cancers [11]. While the exact mechanism of action of EGFR toxicity is not fully elucidated, EGFR is known to be expressed on the ocular surface epithelia, including the cornea, limbus, and conjunctiva [12], and EGFR-mediated processes are involved in eyelash growth and conjunctival epithelial proliferation [13]. EGFR modulates many cell-signaling pathways that cause cellular proliferation, regeneration, and differentiation. Therefore, blocking of these pathways in normal ocular cells may lead to the development of ocular TRAEs, including keratopathy, conjunctivitis, dry eye, visual disturbances, and trichomegaly.
Corneal and Conjunctival Toxicity:
Corneal toxicity of the outermost epithelia is reported in all EGFR inhibitors [14], and keratitis caused by EGFR inhibitors has been termed ‘tyrosine kinase keratitis’ [15]. While most corneal TRAEs can be resolved with treatment discontinuation, erlotinib has been associated with persistent corneal ulceration ,[16] and cetuximab has been found to be associated with non-healing corneal ulcers leading to infectious keratitis [17][18]. Patients should be warned about ocular symptoms such as dry eye and blurred vision that should lead to prompt referral to an ophthalmologist. While conservative treatment such as lubricating dry-eye drops can sometimes resolve symptoms, discontinuing treatment is the definitive cure for corneal epithelial defect [16]. Conjunctivitis is an ocular TRAE associated with EGFR inhibitors. It has been reported to occur in 1.4-14.5% of patients treated with gefitinab [19][20], 19% of patients treated with dacomitinib [21], and 3.3% of patients treated with afatinib [22]. For these patients, no additional treatment or mild dose reduction was needed for symptom resolution; no patients needed permanent treatment discontinuation.
Periorbital Toxicity:
Trichomegaly has been associated with many EGFR inhibitors, including panitumumab, afatinib, gefitinb, erlotinib, and cetuximab [23][24][25][26]. Trichomegaly is particularly a common side effect with erlotinib with incidence rates of 9-32% [14]. Trichomegaly can be treated conservatively with eyelid trimming and good ocular hygiene; if trichiasis occurs, prompt referral to the ophthalmologist is warranted.
Periorbital edema, rash, and blepharitis are also reported ocular TRAEs associated with EGFR inhibitors. Periorbital rash was seen in 37.5% of patients treated with cetuximab [14]. In 3.3% of patients treated with afatinib, periorbital edema and conjunctivitis occurred [22]. Although very rare, 2 cases of cicatricial ectropion have also been reported in association with panitumumab [27][28].
Anterior Uveitis:
Rare cases of uveitis have been reported as an ocular side effect for afatinib and erlotinib [29][30][31]. In all cases uveitis was treated with drug discontinuation. In one case, restart of erlotinib led to the recurrence of bilateral anterior uveitis leading to permanent discontinuation of the drug [31]. Referral to a uveitis specialist is warranted for treatment of acute episodes.
Human Epidermal Growth Factor 2 (HER2) Inhibitors
HER2 is an antigen upregulated in approximately 20% of breast cancers and with the development of HER2 inhibitors, prognosis has significantly improved. The first HER2 inhibitors approved by the FDA were trastuzumab and pertuzumab. The newer class of HER2 drugs includes margetuximab, tucatinib, neratinib, and lapatinib. There are also two FDA approved HER2 antibody drug conjugates: trastuzumab-emtansine and trastuzumab-deruxtecan which are more commonly associated with ocular TRAEs.
In clinical trials, 31.3% of patients treated with trastuzumab-emtansine had ocular TRAEs. The majority of these toxicities were Common Terminology Criteria for Adverse Events grade 1-2 and included dry eye, tearing, and conjunctivitis [32]. HER2 is expressed in corneal epithelial cells which may lead to the side effects of dry eye, blurred vision, and increased lacrimation [33]. Only trastuzumab has been associated with one case of bilateral macular edema and ischemia leading to severe vision loss [34].
Selective Estrogen Receptor Modulators (SERM)
Selective estrogen receptor modulators (SERM) are a class of drugs used to treat hormone estrogen receptor positive breast cancers, osteoporosis, and postmenopausal symptoms. SERMs have tissue-specific agonist and antagonist effects on estrogen receptors, thus leading to different toxicities [35]. There are three FDA approved SERMs: tamoxifen, raloxifene, and toremifene. Tamoxifen and toremifene are reported to have associated ocular TRAEs.
Tamoxifen:
Mentioned in Drug Induced Maculopathy article
Toremifene:
Toremifene is reported to have a 3.7%-10% incidence of cataracts and a 9% incidence of dry eye [36]. Cataracts can be treated with surgical lens replacement while dry eye symptoms can be treated with lubricating eye drops. Keratopathy has been reported with an incidence of 3%; there were no long-term adverse effects of these corneal findings after 3.5 years of follow-up [37].
Aromatase Inhibitors
Aromatase Inhibitors are used to decrease estrogen levels in post-menopausal women by blocking the aromatase enzyme and are used in the treatment of estrogen receptor positive breast cancer. Common aromatase inhibitors include anastrozole, exemestane, and letrozole. The most common ocular adverse effect associated with aromatase inhibitors are mild to moderate dry eye symptoms [38]. Aromatase inhibitors are thought to impact the ocular surface and cause meibomian gland dysfunction. There have been several case series that propose an onset of “de novo'' Sjogren Syndrome in patients with hormone positive breast cancer treated with anastrozole and letrozole [39]. Aromatase inhibitors have also caused adverse effects at the level of the retina. There are subclinical adverse effects including a lower thickness of the retinal nerve fiber layer in patients taking aromatase inhibitors compared to age-matched controls [40]. There have been a few cases reported of bilateral optic neuritis [41] and uveitis associated with the use of aromatase inhibitors [42].
Fibroblast Growth Factor Receptor (FGFR) Inhibitors
FGFR inhibitors, including pemigatinib and erdafitinib, are used in the treatment of cholangiocarcinoma and urothelial carcinoma. The most common ocular adverse effects include those on the ocular surface and retina leading to both dry eye symptoms and retinopathy [43]. These drugs are associated with a serous retinopathy, similar to that seen in MEK inhibitors. This is likely due to commonalities in the FGFR and MEK pathways [44]. Drug induced maculopathy of FGFR inhibitors are mentioned here.
Erdafitinib
In clinical trials 17% of patients needed dose interruption and 6% of patients needed dose discontinuation due to ocular TRAEs [45]. The most severe adverse effects include bilateral retinal detachments and necrotizing keratitis. Minor adverse effects such as dry eye can be treated symptomatically. Dry eye prophylaxis with ocular lubricating eye drops is recommended and comprehensive monthly ophthalmic exams after a baseline exam should be conducted for the first 4 months of treatment [46].
Pemigatinib
Across clinical trials, 6% of patients treated with pemigatinib had retinal pigment epithelial detachments with a median time to onset of 62 days [47]. Dry eye is also a common TRAE. A case of trichomegaly and trichiasis has also been reported after the initiation of pemigatinib [48]. Ophthalmic exams should be performed prior to drug initiation then every 2 months for the first 6 months of treatment, then every 3 months thereafter. In the event of ocular symptoms developing, immediate evaluation by an ophthalmologist is advised.
Infigratinib and futibatinib are other newly-FDA approved FGFR inhibitors that are also associated with similar TRAEs.
Proteasome Inhibitors
Proteasome inhibitors target the ubiquitin-proteasome pathway involved in cellular apoptosis. Dysregulation of this pathway is associated with carcinogenesis. Bortezomib, carfilzomib, and ixazomib are FDA approved proteasome inhibitors used to treat multiple myeloma. Bortezomib is associated with chalazia development with a reported prevalence of 6.8% [49]. Chalazion can be treated with warm compresses, topical antibiotics, and incision and drainage. Blepharitis and meibomitis are also reported with bortezomib, most cases are responsive to conservative treatment and chemotherapy treatment pauses [50]. Carfilzomib has cataracts and blurred vision as FDA-listed side effects [51]. Ixazomib has a 32% incidence of ocular TRAEs in clinical trials with 7% having blurred vision, 7% having conjunctivitis, and 5% dry eye [52]. However, these frequencies were similar to those in placebo groups, and no serious ocular safety concerns were noted with this drug regimen during FDA approval.
BCR-ABL Inhibitors
BCR-ABL inhibitors are tyrosine kinase inhibitors indicated for chronic myeloid leukemia (CML), which is characterized by the Philadelphia chromosome (t(9,22)(q34;q11) balanced reciprocal translocation. The Ph chromosome generates the BCR-ABL oncogene, which codes for the BCR-ABL protein that leads to constitutive kinase activity promoting the growth of leukemic cells [53]. Current drugs on the market are the standard treatment for CML: imatinib, dasatinib, nilotinib, bosutinib, ponatinib, and asciminib [54].
Various ocular adverse effects have been reported with imatinib use, with the most common being periorbital edema and epiphora. Possible toxicities also include extraocular muscle palsies and ptosis. Rarer TRAEs include glaucoma, papilledema, retinal hemorrhage, photosensitivity, abnormal vision, and increased intraocular pressure [49].
Vascular Endothelial Growth Factor Receptor (VEGFR) Inhibitors
Vascular endothelial growth factor (VEGF) and its tyrosine kinase receptor (VEGFR) are blocked via VEGFR anti-neoplastic agents; inhibiting angiogenesis in malignant cells [55]. Currently approved inhibitors include axitinib, cabozantinib, lenvatinib, sunitinib, sorafenib, and vandetanib.
Direct ocular toxicities are rare with VEGFR inhibitors; however, they are associated with systemic adverse effects, such as hypertension that can contribute to visual symptoms. These symptoms include blurry vision and retinopathy [56].
Bruton Tyrosine Kinase (BTK) Inhibitors
Bruton tyrosine kinase (BTK) is essential for B-cell activation, and BTK inhibitors are approved to treat B-cell malignancies such as mantle cell lymphoma. FDA approved BTK inhibitors are ibrutinib, acalabrutinib, zanubrutinib, and most recently, pirtobrutinib.
Overall, these kinase inhibitors are well tolerated, however there have been increasing reports of ibrutinib-associated uveitis and other ocular toxicities [57]. Management recommendations with the development of ophthalmic symptoms includes immediate examination.
JAK2 Inhibitors
Janus kinase (JAK) type 2 inhibitors interfere with the family of JAK proteins, thus disrupting their role in the lymphocytic JAK-STAT signaling pathway [58]. Currently, the most common ophthalmic adverse effect with JAK inhibitors is ophthalmic herpes zoster due to immune compromise [59].
Anaplastic Lymphoma Kinase (ALK) Inhibitors
ALK inhibitors are a class of anti-neoplastic agents primarily used to treat non-small cell lung cancer with the ALK-EML4 gene translocation. Sixty-five percent of patients treated with crizotinib have reported visual disturbances [60]. Visual disturbances include flashes, light trails, and flipped dark-light images in their visual field [61]. These visual changes are benign and usually last less than 1 minute [62]. They frequently dissipate over time with no treatment required. However, more severe TRAEs include visual loss in 0.2% of patients [63], and retinal hemorrhages [64]. The next generation ALK inhibitors including ceritinib, lorlatinib, and brigatinib have similar ocular disturbances although their incidence rates are all much lower, less than 15% [65][66][67].
Mammalian Target of Rapamycin (mTOR) Inhibitors
The mTOR inhibitors are a drug class that targets the mTOR pathway involved in cellular proliferation, angiogenesis, autophagy, and apoptosis [33]. This class of drugs is commonly used to treat metastatic renal cell carcinoma. Temsirolimus has been associated with conjunctivitis, eyelid edema, and excessive lacrimation. The most common ocular TRAEs of everolimus are eyelid edema and conjunctivitis [68].
Cyclin Dependent Kinase (CDK) Inhibitors
CDK inhibitors rarely cause ocular adverse effects. Palbociclib, ribociclib, abemaciclib are some of the FDA approved CDK 4/6 inhibitors used for the treatment of metastatic breast cancer. Palbociclib is associated with increased lacrimation (6.8%), blurred vision (5.5%), and dry eye (4.4%) (69). Ribociclib and abemaciclib are also associated with epiphora (6.9%) and dry eye syndrome [69].
Indoleamine 2,3-dioxygenase (IDO) Inhibitors
Indoleamine 2,3-dioxygenase (IDO) 1 inhibitors block the catalytic reaction needed for oxidation of L-tryptophan into the immunosuppressive L-kynurenine. IDO-1 overexpression allows for cancer immune escape and is associated with poor prognosis [70]. IDO inhibitors are still in clinical trial phases and include Indoximod, Epacadostat, Navoximod, and BMS-986205 [71]. IDO inhibitors can induce the inflammatory signaling pathway through nonspecific activation of AhR or mTOR [72] and have been shown to protect corneal epithelial cells from UV-related damage [73], Epocadostat has been associated with the development of uveitis in a case report [64].
Poly(ADP-ribose) Polymerase (PARP) Inhibitors
Poly(ADP-ribose) polymerase (PARP) is a protein critical to DNA base excision repair for single stranded breaks. There are multiple PARP proteins in this pathway and inhibitors currently target PARP 1 and PARP 2 [74]. Olaparib and talazoparib are PARP inhibitor monotherapies currently approved for germline BRCA-mutated and HER2-negative breast cancer. Ocular adverse effects for olaparib, rucaparib, and niraparib include conjunctivitis, eye swelling, and decreased vision [68].
References
- ↑ Proietti I, Skroza N, Michelini S, Mambrin A, Balduzzi V, Bernardini N, et al. BRAF Inhibitors: Molecular Targeting and Immunomodulatory Actions. Cancers (Basel). 2020;12(7).
- ↑ Kuske M, Westphal D, Wehner R, Schmitz M, Beissert S, Praetorius C, et al. Immunomodulatory effects of BRAF and MEK inhibitors: Implications for Melanoma therapy. Pharmacol Res. 2018;136:151-9.
- ↑ Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21(7):1639-51.
- ↑ Menu E, Kooijman R, Valckenborgh EV, Asosingh K, Bakkus M, Camp BV, et al. Specific roles for the PI3K and the MEK–ERK pathway in IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of multiple myeloma cells: study in the 5T33MM model. British Journal of Cancer. 2004;90(5):1076-83.
- ↑ Jump up to: 5.0 5.1 Méndez-Martínez S, Calvo P, Ruiz-Moreno O, Pardiñas Barón N, Leciñena Bueno J, Gil Ruiz MDR, et al. OCULAR ADVERSE EVENTS ASSOCIATED WITH MEK INHIBITORS. Retina. 2019;39(8):1435-50.
- ↑ Fortes BH, Tailor PD, Dalvin LA. Ocular Toxicity of Targeted Anticancer Agents. Drugs. 2021;81(7):771-823.
- ↑ Jump up to: 7.0 7.1 Stjepanovic N, Velazquez-Martin JP, Bedard PL. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol. 2016;27(6):998-1005.
- ↑ Jump up to: 8.0 8.1 Francis JH, Canestraro J, Haggag-Lindgren D, Harding JJ, Diamond EL, Drilon A, et al. Clinical and Morphologic Characteristics of Extracellular Signal-Regulated Kinase Inhibitor-Associated Retinopathy. Ophthalmology Retina. 2021;5(12):1187-95.
- ↑ Sioufi K, Das S, Say EAT. A Case of Extracellular Signal-Regulated Kinase Inhibitor-Associated Retinopathy. JAMA Ophthalmol. 2020;138(9):1002-4.
- ↑ Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract. 2014;2014:734249.
- ↑ Abourehab MAS, Alqahtani AM, Youssif BGM, Gouda AM. Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism. Molecules. 2021;26(21).
- ↑ Basti S. Ocular toxicities of epidermal growth factor receptor inhibitors and their management. Cancer Nurs. 2007;30(4 Suppl 1):S10-6.
- ↑ Kheir WJ, Sniegowski MC, El-Sawy T, Li A, Esmaeli B. Ophthalmic complications of targeted cancer therapy and recently recognized ophthalmic complications of traditional chemotherapy. Surv Ophthalmol. 2014;59(5):493-502.
- ↑ Jump up to: 14.0 14.1 14.2 Borkar DS, Lacouture ME, Basti S. Spectrum of ocular toxicities from epidermal growth factor receptor inhibitors and their intermediate-term follow-up: a five-year review. Support Care Cancer. 2013;21(4):1167-74.
- ↑ Moshirfar M, Villarreal A, Ronquillo Y. Tyrosine Kinase Inhibitor Keratitis. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
- ↑ Jump up to: 16.0 16.1 Johnson KS, Levin F, Chu DS. Persistent corneal epithelial defect associated with erlotinib treatment. Cornea. 2009;28(6):706-7.
- ↑ Foerster CG, Cursiefen C, Kruse FE. Persisting corneal erosion under cetuximab (Erbitux) treatment (epidermal growth factor receptor antibody). Cornea. 2008;27(5):612-4.
- ↑ Aomatsu K, Sugioka K, Kodama-Takahashi A, Fukuda M, Mishima H, Kusaka S. Corneal Perforation during Combination Chemotherapy including Cetuximab in a Patient with a History of Herpetic Keratitis. Case Rep Oncol Med. 2020;2020:6802408.
- ↑ ullo AB, Esmaeli B, Murray PI, Bristow E, Forsythe BJ, Faulkner K. Ocular findings in patients with solid tumours treated with the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (‘Iressa’, ZD1839) in Phase I and II clinical trials. Eye. 2005;19(7):729-38.
- ↑ Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-46.
- ↑ Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454-66.
- ↑ Jump up to: 22.0 22.1 Seiwert TY, Fayette J, Cupissol D, Del Campo JM, Clement PM, Hitt R, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25(9):1813-20.
- ↑ Matos LV, Pissarra A, Malheiro M, Plácido AN. Trichomegaly of the eyelashes induced by the epidermal growth factor receptor inhibitor cetuximab in the treatment of metastatic colorectal cancer. BMJ Case Rep. 2019;12(4).
- ↑ Maka VV, Rajanna H, Narasiyappah AK, Chitrapur R, Kilara N. Epidermal growth factor receptor inhibitors related trichomegaly of Eyelashes. Oxf Med Case Reports. 2014;2014(5):98-9.
- ↑ Morris LG, Hochster HS, Delacure MD. Eyelash trichomegaly secondary to panitumumab therapy. Curr Oncol. 2011;18(3):145-6.
- ↑ Goel V, Raina S, Chandragouda D, Singh S, Talwar V, Patnaik N. Trichomegaly of eyelashes after treatment with erlotinib in carcinoma pancreas. Int J Trichology. 2014;6(1):23-4.
- ↑ Jin HD, Blessing NW. Cicatricial ectropion and madarosis associated with panitumumab treatment of metastatic colorectal cancer. Am J Ophthalmol Case Rep. 2020;19:100810.
- ↑ Scofield-Kaplan S, Todaro J, Winn BJ. Reversible cicatricial ectropion associated with EGFR inhibitors. Orbit. 2018;37(5):364-7.
- ↑ Klein KA, Azzoli CG, Rifkin LM. Bilateral acute simultaneous onset anterior uveitis presumed secondary to erlotinib: A report of two cases. American Journal of Ophthalmology Case Reports. 2017;6:21-3.
- ↑ Todokoro D, Itakura H, Ibe T, Kishi S. Anterior Uveitis Caused by Ocular Side Effects of Afatinib: A Case Report. Case Rep Ophthalmol. 2016;7(1):74-8.
- ↑ Jump up to: 31.0 31.1 Ali K, Kumar I, Usman-Saeed M, Usman Saeed M. Erlotinib-related bilateral anterior uveitis. BMJ Case Rep. 2011;2011.
- ↑ Burris HA, 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29(4):398-405.
- ↑ Jump up to: 33.0 33.1 Asencio-Durán M, Fernández-Gutiérrez E, Larrañaga-Cores M, Klein-Burgos C, Dabad-Moreno JV, Capote-Díez M. Ocular side effects of oncological therapies: Review. Archivos de la Sociedad Española de Oftalmología (English Edition). 2024;99(3):109-32.
- ↑ Saleh M, Bourcier T, Noel G, Speeg-Schatz C, Gaucher D. Bilateral macular ischemia and severe visual loss following trastuzumab therapy. Acta Oncol. 2011;50(3):477-8.
- ↑ An KC. Selective Estrogen Receptor Modulators. Asian Spine J. 2016;10(4):787-91.
- ↑ FARESTON® (toremifene citrate) [package insert]. F. M. Howell & Company; 1997.
- ↑ Parkkari M, Paakkala AM, Salminen L, Holli K. Ocular side-effects in breast cancer patients treated with tamoxifen and toremifene: a randomized follow-up study. Acta Ophthalmol Scand. 2003;81(5):495-9.
- ↑ Serban D, Costea DO, Zgura A, Tudosie MS, Dascalu AM, Gangura GA, et al. Ocular Side Effects of Aromatase Inhibitor Endocrine Therapy in Breast Cancer - A Review. In Vivo. 2022;36(1):40-8.
- ↑ Guidelli GM, Martellucci I, Galeazzi M, Francini G, Fioravanti A. Sjögren's syndrome and aromatase inhibitors treatment: is there a link? Clin Exp Rheumatol. 2013;31(4):653-4.
- ↑ Moschos MM, Chatziralli IP, Sergentanis T, Zagouri F, Chrysikos D, Ladas I, et al. Electroretinographic and optical coherence tomography findings in breast cancer patients using aromatase inhibitors. Cutan Ocul Toxicol. 2016;35(1):13-20.
- ↑ Coppes OJ, Lukas RV, Fleming GF, Nichols J, Tenney M, Bernard J. Bilateral Optic Disc Swelling Following Anastrozole Therapy. Neuroophthalmology. 2014;38(5):268-71.
- ↑ Sathiamoorthi S, Ruddy KJ, Bakri SJ. Association of Uveitis and Macular Edema With Anastrozole Therapy. JAMA Ophthalmol. 2018;136(7):837-9.
- ↑ Hsu J, Francis JH, Ahmad S. Ocular toxicities of fibroblast growth factor receptor inhibitors: A review. Surv Ophthalmol. 2024;69(1):34-41.
- ↑ Van der Noll R, Leijen S, Neuteboom GH, Beijnen JH, Schellens JH. Effect of inhibition of the FGFR-MAPK signaling pathway on the development of ocular toxicities. Cancer Treat Rev. 2013;39(6):664-72.
- ↑ Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381(4):338-48.
- ↑ BALVERSA (erdafitinib) [package insert]. anssen Biotech, Inc; 2019.
- ↑ Patel TH, Marcus L, Horiba MN, Donoghue M, Chatterjee S, Mishra-Kalyani PS, et al. FDA Approval Summary: Pemigatinib for Previously Treated, Unresectable Locally Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusion or Other Rearrangement. Clin Cancer Res. 2023;29(5):838-42.
- ↑ Campagna GA, Liu ST, Tsui E. Trichomegaly and Trichiasis Associated with Pemigatinib. Ophthalmology. 2021;128(9):1364.
- ↑ Jump up to: 49.0 49.1 Fraunfelder FW, Yang HK. Association Between Bortezomib Therapy and Eyelid Chalazia. JAMA Ophthalmology. 2016;134(1):88-90.
- ↑ Puri S, Joshi J, Derman O, Kornblum N, Verma A, Braunschweig I, et al. Ocular Complications of Bortezomib Therapy in Multiple Myeloma. Blood. 2014;124(21):5743.
- ↑ KYPROLIS® (carfilzomib) [package insert]. Onyx Pharmaceuticals, Inc; 2012.
- ↑ Kumar S, Moreau P, Hari P, Mateos MV, Ludwig H, Shustik C, et al. Management of adverse events associated with ixazomib plus lenalidomide/dexamethasone in relapsed/refractory multiple myeloma. Br J Haematol. 2017;178(4):571-82.
- ↑ Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7(6):441-53.
- ↑ Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93(3):442-59.
- ↑ Zhong H, Bowen JP. Molecular design and clinical development of VEGFR kinase inhibitors. Curr Top Med Chem. 2007;7(14):1379-93.
- ↑ Davis ME. Ocular Toxicity of Tyrosine Kinase Inhibitors. Oncol Nurs Forum. 2016;43(2):235-43.
- ↑ Chiu ZK, Goh JK, Ling C, Lin ML, Hall AJ. Ibrutinib-related uveitis: A case series. Am J Ophthalmol Case Rep. 2022;25:101300.
- ↑ Lin CM, Cooles FA, Isaacs JD. Basic Mechanisms of JAK Inhibition. Mediterr J Rheumatol. 2020;31(Suppl 1):100-4.
- ↑ Dammacco R, Guerriero S, Alessio G, Dammacco F. Natural and iatrogenic ocular manifestations of rheumatoid arthritis: a systematic review. Int Ophthalmol. 2022;42(2):689-711.
- ↑ Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-703.
- ↑ Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011-9.
- ↑ Kazandjian D, Blumenthal GM, Chen HY, He K, Patel M, Justice R, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5-11.
- ↑ Chun SG, Iyengar P, Gerber DE, Hogan RN, Timmerman RD. Optic neuropathy and blindness associated with crizotinib for non-small-cell lung cancer with EML4-ALK translocation. J Clin Oncol. 2015;33(5):e25-6.
- ↑ Jump up to: 64.0 64.1 Parikh RA, Chaon BC, Berkenstock MK. Ocular Complications of Checkpoint Inhibitors and Immunotherapeutic Agents: A Case Series. Ocul Immunol Inflamm. 2021;29(7-8):1585-90.
- ↑ ALUNBRIG® (brigatinib) [package insert]. ARIAD Pharmaceuticals, Inc; 2017.
- ↑ LORBRENA® (lorlatinib) [package insert]. Pfizer Oncology Together; 2018.
- ↑ Raedler LA. Zykadia (Ceritinib) Approved for Patients with Crizotinib-Resistant ALK -Positive Non-Small-Cell Lung Cancer. Am Health Drug Benefits. 2015;8(Spec Feature):163-6.
- ↑ Jump up to: 68.0 68.1 Sakellakis M, Spathas N, Tsaousis KT, Nikitiadis EN, Linardou H, Diakonis VF. Potential Ophthalmological Side Effects Induced by Anti-Neoplastic Regimens for the Treatment of Genitourinary Cancers: A Review. Cureus. 2022;14(7):e27266.
- ↑ Canino F, Omarini C, Cerma K, Moscetti L, Tornincasa A, Trudu L, et al. Ocular Toxicity in Breast Cancer Management: Manual for The Oncologist. Clinical Breast Cancer. 2022;22(4):289-99.
- ↑ Tang K, Wu Y-H, Song Y, Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. Journal of Hematology & Oncology. 2021;14(1):68
- ↑ Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9(1):1777625.
- ↑ Günther J, Däbritz J, Wirthgen E. Limitations and Off-Target Effects of Tryptophan-Related IDO Inhibitors in Cancer Treatment. Front Immunol. 2019;10:1801.
- ↑ Beutelspacher SC, Tan PH, McClure MO, Larkin DF, Lechler RI, George AJ. Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses. Am J Transplant. 2006;6(6):1320-30.
- ↑ Chen A. PARP inhibitors: its role in treatment of cancer. Chin J Cancer. 2011;30(7):463-71.

