Ocular Surface Adverse Events and Changes Related to Antibody-Drug Conjugates
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
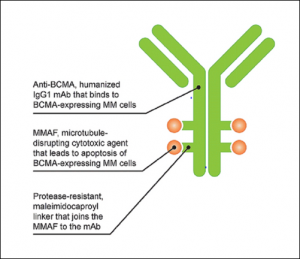
ADCs are a novel class of drugs used for the treatment of certain cancers resistant to traditional therapies. These drugs consist of a tumor-specific monoclonal antibody (MAB) linked to a cytotoxic payload. The MAB component attaches to its tumor-specific antigen on cancer cells, and the entire ADC is endocytosed. The cytotoxic payload is then cleaved by lysosomal enzymes within cancer cells. This activates a cascade of events resulting in cellular apoptosis. [1][2] (Figure 1) Ocular surface toxicity is one of the most common adverse events (AE) associated with ADCs.
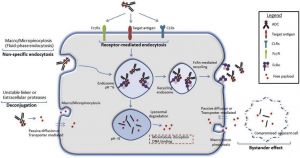
Two proposed mechanisms for ADC-mediated damage are on-target versus off-target toxicity. On-target toxicity may occur via receptor-mediated endocytosis in ocular surface cells that express the target antigen for a specific ADC. Off-target toxicity may occur through Fc-receptor mediated entry, macropinocytosis of the ADC (also known as “cell drinking”), or bystander toxicity due to premature cleavage of the linker in the bloodstream and liberation of the cytoxic payload. [3][4] Passive diffusion of the cytotoxic payload through the permeable cell membrane could also cause off-target toxicity.[3] (Figure 2)
Currently Approved Medications
Table 1. FDA approved ADCs as of April 2023[5][6]
| Drug | Trade name | target | General Indication |
| Gemtuzumab[AF1] ozogamicin | Mylotarg | CD33 | CD33+ relapsed or refractory AML |
| Brentuximab vedotin | Adcetris | CD30 | Lymphoma |
| Adotrastuzumab emtansine | Kadcyla | HER2 | HER2+ breast cancer |
| Inotuzumab ozogamicin | Besponsa | CD22 | Relapsed or refractory B-ALL |
| Polatuzumab vedotin-piiq | Polivy | CD79b | Relapsed or refractory DLBCL |
| Enfortumab vedotin-ejfv | Padcev | Nectin4 | Urothelial cancer |
| Famtrastuzumab deruxtecan-nxki | Enhertu | HER2 | HER2+ breast cancer; HER2+ gastric or gastroesophageal junction adenocarcinoma |
| Sacituzumab govitecan-hziy | Trodelvy | TROP2 | Metastatic triple-negative breast cancer; urothelial cancer |
| Loncastuximab tesirine-lpvl | Zynlonta | CD19 | Large B-cell lymphoma |
| Belantamab mafodotin | Blenrep | BCMA | Relapsed or refractory multiple myeloma.
*Withdrawn in 2022 because confirmatory phase 3 trial did not meet primary efficacy endpoint
|
| Tisotumab vedotin-tftv | Tivdak | TF | Recurrent or metastatic cervical cancer |
| Mirvetuximab soravtansine | Elahere | FR-alpha | Platinum-resistant ovarian cancer |
| Moxetumomab Pasudotox | Lumoxiti | CD-22 | Hairy cell leukemia |
| Disitamab Vedotin | Aidixi | HER2 | Gastric carcinoma, Urothelial carcinoma |
Reported Adverse Events
Pseudomicrocysts
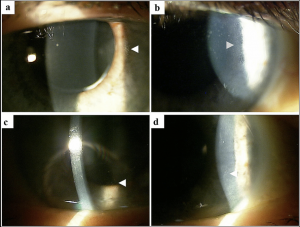
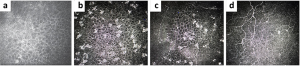
a. Disease entity: ADCs lead to the formation of corneal epithelial inclusions that begin near the limbus and extend centrally as drug dosage and duration of therapy increase (Figure 3). These changes occur bilaterally and are termed “pseudomicrocysts” or “microcyst-like” because they are not felt to represent true microcysts.[1] In vivo confocal microscopy (IVCM) has demonstrated the presence of round, hyperreflective intracellular structures most prominent in the basal layer of the epithelium with relative sparing of the superficial epithelium. (Figure 4) It is unclear what these hyperreflective structures are, but one leading hypothesis is that they represent the internalization or phagocytosis of ADCs by corneal epithelial cells.[1][7] No material is seen within the stroma or endothelium. These corneal changes have been reported with the use of multiple ADCs including belantamab mafodotin and mirvetuximab soravtansine.[1][8]
b. Presentation: Patients may complain of symptoms such as irritation, blurred vision, tearing, and photophobia, although often patients are asymptomatic. Diminished visual acuity and subjective feelings of blurred vision may be correlated with more centrally located pseudomicrocysts.[1][8][9][10] Both hyperopic and myopic shifts have been observed and correlated to their location.[11]
c. Risk Factors: Patients with a history of dry eye prior to starting a course of ADCs may be predisposed to the development of pseudomicrocysts.[1]
d. Histopathology: In a recent case series by Kreps et al. of two patients who developed pseudomicrocysts from an ADC used to treat glioblastoma multiforme, corneal scrapings from the two patients were examined microscopically. Histology revealed epithelial cells with a vacuolated, granular appearance at various stages of apoptosis. Basal epithelial cells and engulfed apoptotic cells stained positive for IgG on immunohistochemistry.[12] These findings suggest that there is a mechanism by which ADCs are internalized by corneal epithelial cells and exert their cytotoxic effects.
e. Pathophysiology: Corneal epithelial cells theoretically undergo ADC-induced cell death, facilitating further (peripheral) pseudomicrocysts that migrate centrally as new epithelial cells are produced. It is unclear whether ADCs enter the cornea through the vascularized limbus or through the tear film, although their pattern of migration suggests the former.[1] In the cases of belantamab mafodotin and mirvetuximab soravtansine, the target is not expressed in corneal or conjunctival epithelial cells. Thus, an off-target pathway is suspected.[1][13]
Conjunctivitis
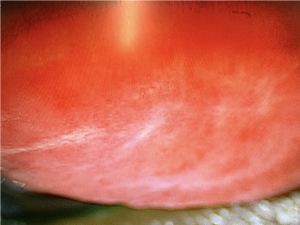
Conjunctivitis typically presents with bilateral bulbar and/or palpebral hyperemia and complaints of burning, itching, tearing. Patients may have concurrent blepharitis or eyelid irritation. Conjunctivitis is a reported side effect of tisotumab. Subepithelial fibrosis has also been observed. (Figure 5)
Limbal stem cell dysfunction
Patients may present with worsening of dry eye symptoms. Staining with fluorescein demonstrates a whorl pattern staining typical of limbal stem cell dysfunction.
Superior limbic keratoconjuctivitis
Superior limbic keratoconjunctivitis has been observed with the use of mirvetuximab soravtansine.
Management
As with most patients who are on anti-cancer therapies with potential ocular side effects, the mainstays of management revolve around routine follow-up, dose modification, and supportive care. Avoidance of contact lens wear may also reduce surface toxicity. Punctal plugs may increase the concentration or exposure of the ocular surface to these medications as well.
Routine eye exams
Patients should get baseline examinations prior to initiation of medications with potential ophthalmic toxicity and be monitored every cycle or sooner with increased symptoms. The KVA scale can be used to grade the level of keratopathy.
Medical therapy
Patients, particularly those with dry eye upon baseline examination, are advised to start preservative-free artificial tears four times a day in both eyes.[1] Prophylactic artificial tears should be prescribed for patients starting enfortumab and tisotumab.[14] [15] Prophylactic topical corticosteroids have been used with mixed results.[2][8][16] They have been suggested to be effective in mitigating ocular adverse events associated with the ADCs depatuxizumab mafodotin (ABT-414) and vorsetuzumab mafodotin (SGN-75), both of which contain the cytoxic payload monomethyl auristatin F (MMAF). [2][8][17][18] Steroid prophylaxis with 1% prednisolone 4-6 times daily during the first 10 days of a treatment cycle of mirvetuximab soravtansine appeared to reduce the incidence of keratopathy.[13] Long-term steroid treatment should be used with caution due to risk of secondary infection as well as usual risk of ocular hypertension, glaucoma and cataract. Other treatments that are typically used for patients with severe ocular surface disease such as vitamin A ointment and oral doxycycline (20 mg PO BID) may also be beneficial.
Dose Modification
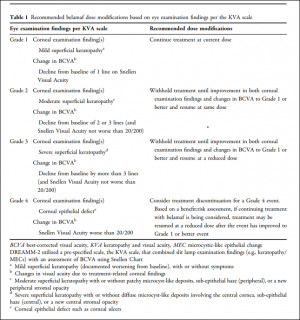
In the presence of ocular surface adverse events, it may be advisable to delay treatment and reduce the dose of the medication. For example, dose delays and reductions have been shown to reduce MECs in the DREAMM-2 study which was a phase 2 study of the ADC belantamab mafodotin used for multiple myeloma.[19]Table 2 shows the recommended dose modifications for belantamab mafodotin based on the keratopathy visual acuity (KVA) scale, which is composed of corneal findings and change in BCVA. Corneal changes from this and other MMAF-containing ADCs were reversible with treatment cessation, often showing resolution from a few weeks to several months.[2][16][18][19][20][21] Similar dose modifications have also been recommended for patients undergoing treatment with mirvetuximab soravtansine, as outlined by Hendershot et al.[22] Dose modifications and delays are also recommended for tisotumab in presence of keratitis or conjunctivitis more severe than Grade 1. Discontinuation is recommended in the setting of severe keratitis with ulceration, Grade 3 or 4 conjunctivitis, and any sign of corneal or conjunctival scarring or symblepharon. [15]
Prognosis
Awareness of ocular surface adverse events, with appropriate monitoring and treatment is key for patient outcomes. The symptoms and ocular findings typically resolve with cessation of the medication with good visual recovery. Pseudomicrocysts largely resolve with discontinuation or tapering of the offending agent as pseudomicrocyst-containing cells are replaced with healthy, regenerated cornea epithelium. Resolution of symptoms and regeneration of the corneal epithelium can vary from weeks to months.[1] Conjunctivitis, such as that caused by agents like tisotumab, can result in long term conjunctival scarring.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Farooq, A.V., Degli Esposti, S., Popat, R. et al. Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody–Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study. Ophthalmol Ther (2020).
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Tannir NM, Forero-Torres A, Ramchandren R, et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs. 2014;32:1246–57.
- ↑ Jump up to: 3.0 3.1 De Goeij BE, Lambert JM. New developments for antibody-drug conjugate-based therapeutic approaches. Curr Opin Immunol. 2016;40:14–23.
- ↑ Mahalingaiah PK, Ciurlionis R, Durbin KR, et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019;200:110–25.
- ↑ Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2021;26. doi:10.3390/molecules26195847
- ↑ Mühlegger M. FDA approved antibody-drug conjugates for cancer therapy. [cited 16 Aug 2023]. Available: https://www.susupport.com/knowledge/bioconjugates/fda-approved-antibody-drug-conjugates
- ↑ Lee BA, Lee MS, Maltry AC, Hou JH. Clinical and histological characterization of toxic keratopathy from depatuxizumab mafodotin (ABT-414), an antibody-drug conjugate: 2021 Sep 1;40(9):1197-1200
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Corbelli E, Miserocchi E, Marchese A, et al. Ocular toxicity of mirvetuximab. Cornea. 2019;38:229–32.
- ↑ Deklerck E, Denys H, Kreps EO. Corneal features in trastuzumab emtansine treatment: not a rare occurrence. Breast Cancer Res Treat. 2019;175:525–30.
- ↑ Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31:589–604.
- ↑ Canestraro J, Hultcrantz M, Modi S, Hamlin PA, Shoushtari AN, Konner JA, et al. Refractive Shifts and Changes in Corneal Curvature Associated With Antibody–Drug Conjugates. Cornea. 2022;41: 792.
- ↑ Kreps EO, Derveaux T, Denys H. Corneal changes in trastuzumab emtansine treatment. Clin Breast Cancer. 2018;18:e427–e429429.
- ↑ Jump up to: 13.0 13.1 Matulonis UA, Birrer MJ, O’Malley DM, Moore KN, Konner J, Gilbert L, et al. Evaluation of Prophylactic Corticosteroid Eye Drop Use in the Management of Corneal Abnormalities Induced by the Antibody–Drug Conjugate Mirvetuximab Soravtansine. Clin Cancer Res. 2019;25: 1727–1736.
- ↑ Mukhtar S, Jhanji V. Effects of systemic targeted immunosuppressive therapy on ocular surface. Curr Opin Ophthalmol. 2022;33: 311–317.
- ↑ Jump up to: 15.0 15.1 Arn CR, Halla KJ, Gill S. Tisotumab Vedotin Safety and Tolerability in Clinical Practice: Managing Adverse Events. J Adv Pract Oncol. 2023;14: 139–152.
- ↑ Jump up to: 16.0 16.1 Gan HK, Reardon DA, Lassman AB, Merrell R, van den Bent M, Butowski N, et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro Oncol. 2018;20: 838–847.
- ↑ Goss GD, Vokes EE, Gordon MS, Gandhi L, Papadopoulos KP, Rasco DW, et al. Efficacy and safety results of depatuxizumab mafodotin (ABT-414) in patients with advanced solid tumors likely to overexpress epidermal growth factor receptor. Cancer. 2018;124: 2174–2183.
- ↑ Jump up to: 18.0 18.1 van den Bent M, Gan HK, Lassman AB, Kumthekar P, Merrell R, Butowski N, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol. 2017;80: 1209–1217.
- ↑ Jump up to: 19.0 19.1 Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21: 207–221.
- ↑ Younes A, Kim S, Romaguera J, Copeland A, Farial S de C, Kwak LW, et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J Clin Oncol. 2012;30: 2776–2782.
- ↑ Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20: 1124–1135.
- ↑ Hendershot A, Slabaugh M, Riaz KM, Moore KN, O’Malley DM, Matulonis U, et al. Strategies for prevention and management of ocular events occurring with mirvetuximab soravtansine. Gynecol Oncol Rep. 2023;47: 101155.