Retinal Astrocytic Hamartoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Retinal astrocytic hamartomas (sometimes called retinal astrocytoma) are benign glial cell tumors. They are often encountered as an asymptomatic lesion in screening of patients with tuberous sclerosis complex, but may be sporadic. Diagnosis is largely clinical and may be supported by ancillary tests. In tuberous sclerosis, retinal findings are significantly associated with concurrent neurological and renal disease. Growth or complications of RAH requiring treatment are rare.
Disease Entity
Retinal astrocytic hamartomas (RAH) are benign retinal tumors composed of glial cells. They are typically encountered as an asymptomatic lesion in patients with tuberous sclerosis complex (TSC); however, may be isolated tumors or found in association with other ocular or systemic disease.[1][2] Most RAH are thought to be congenital, but may not become clinically apparent until later in life.
Disease
RAH are not specifically recognized in the International Classification of Diseases (ICD) version 10 nomenclature.
- D31.2 Benign neoplasm: Retina
- Q85.1 Tuberous sclerosis
Etiology
RAH were first recognized in 1921 by Van der Hoeve, in association with TSC.[3] He termed the lesions ‘phakomas’ (from the Greek phakos meaning ‘spot’). RAH are found in one third to one half of patients with TSC. Approximately one third of these patients will have bilateral or multiple tumors.[4][5]
Sporadic RAH (those with no clinical or genetic evidence of TSC) are almost always solitary and unilateral. They are estimated to comprise between 29% to 81% of total RAH.[1][6]
Associations
The most frequent association of RAH is with TSC. Other associations have been reported, including:
- Neurofibromatosis (type 1)
- Retinitis pigmentosa[7][8][9]
- Usher’s syndrome[10]
- Stargardt’s disease / ABCR (ABCA4) mutation[11]
- Gyrate atrophy[12]
General Pathology
A hamartoma (from the Greek hamartanein meaning ‘to err’) is a disorganised overgrowth of benign cells within their native tissue. RAH are hamartomas composed of elongated fibrous glial cells (mostly astrocytes), which have small uniform nuclei and interlacing cytoplasmic processes. Other histological features include foci of dystrophic calcification, and large pleomorphic gemistocytes.[2][13][14]
Pathophysiology
TSC is caused by mutation of either TSC1 or TSC2, tumor suppressor genes that encode for the proteins hamartin and tuberin, respectively. Loss of function leads to dysregulation of cellular proliferation via the mTOR (mammalian target of rapamycin) pathway, resulting in hamartoma formation in multiple organs. Tumor formation relies on a second random mutation (Knudson’s ‘two hit’ hypothesis), accounting for the wide phenotypic variation.[15]
TSC has an autosomal dominant inheritance pattern, with high penetrance but widely variable expressivity.[15] Approximately 2/3 of cases are sporadic, but may then be inherited by subsequent generations.
Diagnosis
RAH are diagnosed clinically based on characteristic ophthalmoscopic appearance.[2][5] Ancillary testing may be of value in establishing a diagnosis in patients with atypical appearing lesions or those without tuberous sclerosis.[1]
Symptoms
RAH are typically asymptomatic, and may be detected incidentally or on screening of patients with suspected phakomatoses. Macular or optic nerve tumors, or those complicated by hemorrhage or exudation, may cause visual disturbance (blur, floaters, or metamorphopsia).
Signs
Classically, three morphological subtypes of RAH have been described.[4][13] These are:
- Type 1 (most common): relatively flat, smooth, semitransparent, grey-white lesions without calcification
- Type 2: raised, multinodular (‘mulberry-like’), opaque, calcified lesions
- Type 3: transitional lesion with features of Type 1 and Type 2
Lesions typically remain stable with age, and do not evolve between types.[4]
Practically, tumors can be divided into calcified and non-calcified variants. The characteristic feature of calcified tumors is glistening yellow spherules of calcification. This differs from the chalky, dull calcification seen in retinoblastoma.[2] RAH also lack a prominent feeding/draining vessel, in contrast to retinoblastoma or retinal capillary hemangioma.
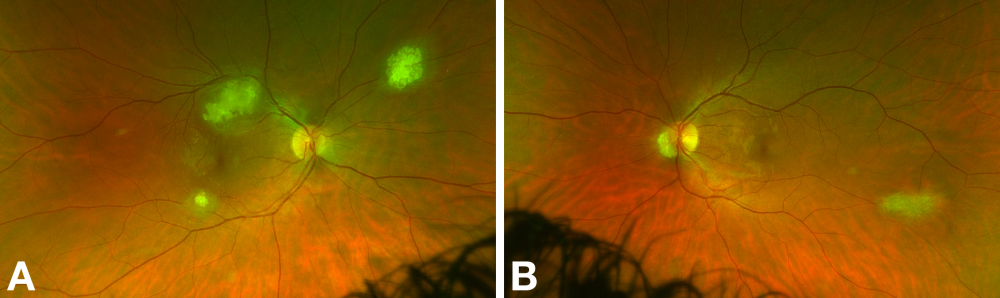
In a large series of patients from a tuberous sclerosis program by Aronow et al., 36% had RAH.[5] There was a significant prevalence of bilateral (43%) and multiple (40%) tumors. In this series, average basal tumor diameter was one disc diameter (range, 0.5-2.5 disc diameters), and the average number of RAH was four (range, 2-7).
Tuberous Sclerosis Complex
TSC is a phakomatosis, with widely variable manifestations depending on the severity and extent of involvement in various organ systems (eyes, brain, heart, lungs, kidneys, skin).
Other ocular signs associated with TSC include[4]:
- ‘Punched out’ depigmented retinal pigment epithelial lesions[16]
- Angiofibromas of the eyelids
- Poliosis
- Iris or chorioretinal coloboma
- Sectoral iris heterochromia
- Papilledema
- Strabismus
TSC patients with retinal findings are more likely to have concurrent systemic disease compared to those with a normal ophthalmoscopic examination. Aronow et al. found that patients with retinal changes were three times more likely to have concurrent giant cell astrocytomas, and significantly more likely to describe cognitive impairment, epilepsy, and renal angiomyolipomas compared to those without.[5]
Systemic signs associated with tuberous sclerosis complex include:
- Skin signs
- Ash leaf spots (depigmented macules – may require a ‘Wood’s lamp’ ultraviolet light in fair-skinned individuals)
- Shagreen patch (thick leathery dimpled patches)
- Adenoma sebaceum (cutaneous angiofibromas in malar distribution)
- Ungual fibromas
- Gingival fibromas
- Neurological signs
- Seizures / infantile spasms
- Intellectual disability
- Neuropsychiatric (developmental, behavioural, or psychosocial difficulties)
- Cortical tubers
- Giant cell astrocytoma (may cause obstructive hydrocephalus)
- Subependymal nodules (in the walls of ventricles)
- Other organ manifestations
- Cardiac rhabdomyoma (may cause cardiac arrhythmias)
- Renal angiomyolipoma (may cause significant renal hemorrhage and hematuria)
- Lymphangioleiomyomatosis (LAM, cystic replacement of the lung parenchyma)
TSC clinical diagnostic criteria have been defined by the International Tuberous Sclerosis Complex Consensus Group in 2012.[15]
| Updated Diagnostic Criteria for Tuberous Sclerosis Complex, 2012
Courtesy of H. Northrup, D.A. Krueger on behalf of the International Tuberous Sclerosis Complex Consensus Group |
|---|
| A. Genetic diagnostic criteria
|
| B. Clinical diagnostic criteria Major features 1. Hypomelanotic macules (≥3, at least 5mm diameter) 2. Angiofibromas (≥3) or fibrous cephalic plaque 3. Ungual fibromas (≥2) 4. Shagreen patch 5. Multiple retinal hamartomas 6. Cortical dysplasias* 7. Subependymal nodules 8. Subependymal giant cell astrocytoma 9. Cardiac rhabdomyoma 10. Lymphangioleiomyomatosis (LAM) 11. Angiomyolipomas** (≥2) Minor features 1. “Confetti” skin lesions 2. Dental enamel pits (>3) 3. Intraoral fibromas (≥2) 4. Retinal achromic patch 5. Multiple renal cysts 6. Nonrenal hamartomas |
| Definite diagnosis: Two major features or one major feature with ≥2 minor features
Possible diagnosis: Either one major feature or ≥2 minor features *Includes tubers and cerebral white matter radial migration lines. **A combination of the two major clinical features (LAM and angiomyolipomas) without other features does not meet criteria for a definite diagnosis. |
Diagnostic procedures
Ancillary (in clinic) testing may assist in diagnosis.
Optical Coherence Tomography
Spectral-domain (SD) optical coherence tomography (OCT) features of RAH are well described.[1][17][18][19] Tumors are localized in the retinal nerve fiber layer, with dome-shaped thickening and hyperreflectivity present at the inner retina. Characteristic optically empty spaces (‘moth eaten’ areas) are present representing intralesional calcification or cavitation. Outer retinal layers may be compressed or disorganised by the tumor. Calcification and may cause posterior optical shadowing. OCT may also demonstrate CNVM.[20]
An OCT classification system for RAH in TSC has been proposed, with four subtypes defined by OCT criteria.[21] This study reported that thicker and calcified tumors are significantly more likely to have associated systemic disease.
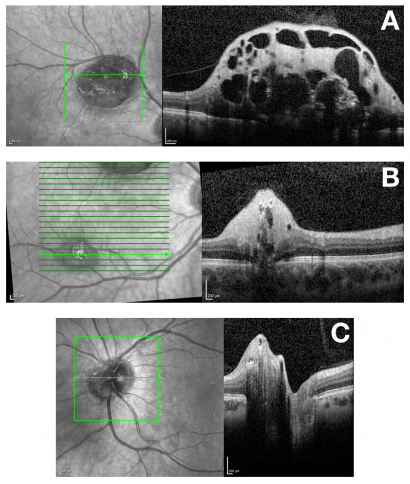
OCT Angiography
OCT angiography demonstrates a dense vascular network within the tumor in the superficial and deep retinal plexi, with flow voids corresponding to areas of cavitation.[22][23]
Ultrasound
B-mode ultrasonography shows an acoustically solid tumor, and may demonstrate hyperechogenic calcifications with posterior shadowing.[9][22]
Fundus Autofluorescence
Non-calcified RAH reduce the physiological background autofluorescence. Calcified tumors demonstrate hyperautofluorescence.[13]
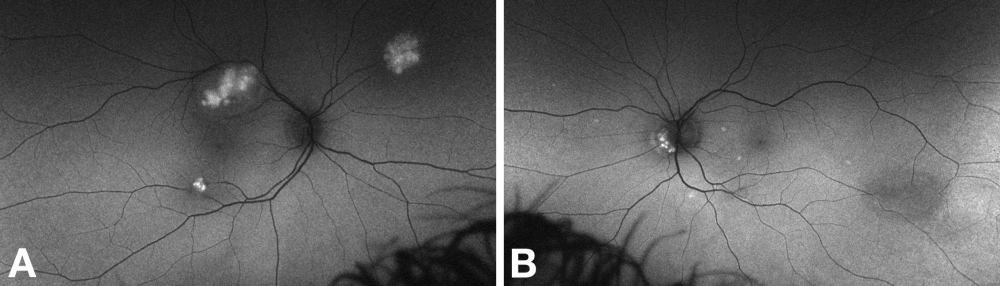
Fluorescein Angiography
Fundus fluorescein angiography demonstrates early blockage of background choroidal fluorescence, a plexus of capillaries within the tumor, and late leak (hyperfluorescence, gradually increasing in intensity and extent).[13][24] Angiography may demonstrate additional tumors that are not apparent clinically.
Indocyanine Green Angiography
Indocyanine green angiography demonstrates hypocyanescence due to masking by the tumors.[24] Degree of calcification and height of the tumor determine the amount of blockage.[13]
Laboratory test
Biopsy is rarely required and not typically recommended for RAH. Fine-needle aspiration for cytopathology can however be performed in atypical cases.[25][26] Cytology demonstrates spindle cells, and immunohistochemistry staining is positive for glial cells (glial-fibrillary acidic protein stain).
TSC is a clinical diagnosis, but genetic testing for TSC1 and TSC2 mutations is available.
Differential diagnosis
Differential diagnoses include[2]:
- Solitary circumscribed retinal astrocytic proliferation
- Acquired retinal astrocytoma
- Solitary mass similar to RAH but lacking calcification, of adult onset in patients with no history of tuberous sclerosis
- Retinoblastoma
- Retinocytoma
- Uveal melanoma
- May demonstrate cavitations, or rarely calcification[29]
- Choroidal metastasis (in particular from carcinoid tumor)
- Combined hamartoma of the retina and retinal pigment epithelium
- Retinal pigment epithelial adenoma
- Retinal capillary hemangioma
- Optic nerve head drusen[9]
- Anterior drusen typically demonstrate hyperautofluorescence
OCT and OCT angiography features of choroidal and retinal tumors are well described elsewhere.[22][30]
Complications
Complications are uncommon, but include:
- Retinal traction[1]
- Choroidal neovascular membrane[20]
- Cystoid macular edema[31]
- Exudative retinal detachment[32]
- Vitreous hemorrhage[31]
- Subretinal hemorrhage[33]
- Neovascular glaucoma[34]
- Amblyopia
Management
Most RAH are small and non-progressive, and periodic monitoring is typically all that is required. Serial ophthalmoscopic examination is necessary to detect those lesions showing aggressive growth behaviour or vision-threatening complications. TSC patients, particularly if young or cognitively impaired, may be less likely to report symptoms.
Medical therapy
TSC patients may require treatment of subependymal giant cell astrocytomas with mTOR inhibitors (eg everolimus, sirolimus).[35] mTOR inhibitors have been shown to cause regression of RAH[36][37], and have been used for aggressive ocular disease in a child.[38]
Treatment may be required for the rare complications associated with RAH. Intravitreal ranibizumab has been reported to be effective for CNVM, achieving closure and resolution of fluid after a short course of injections.[20] Laser photocoagulation may be effective for exudative retinal detachments that do not resolve spontaneously, but prognosis for improvement in vision is guarded. Low fluence, direct laser treatments are advocated to reduce the risk of exacerbating exudation.[32]
Surgery
Vitrectomy may be necessary for hemorrhage or exudation not amenable to non-operative measures.[2][39]
Prognosis
RAH usually remain stable throughout life. Progressive growth and local complications (see above) are uncommon.[2]
Additional Resources
- TSC Alliance (USA)
- Tuberous Sclerosis Association (UK)
- Tuberous Sclerosis Australia (Aus)
- Tuberous Sclerosis – National Organization for Rare Disorders (NORD)
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Shields CL, Say EAT, Fuller T, Arora S, Samara WA, Shields JA. Retinal Astrocytic Hamartoma Arises in Nerve Fiber Layer and Shows “Moth-Eaten” Optically Empty Spaces on Optical Coherence Tomography. Ophthalmology. 2016;123(8):1809-16.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Shields JA, Shields CL. Intraocular tumors : an atlas and textbook. Place of publication not identified: Wolters Kluwer Health,; 2015.
- ↑ Van der Hoeve T. Augengeschwülste bei der tuberösen Hirnsklerose (Bourneville). Albrecht Von Graefes Arch Ophthalmol. 1921(105):880-98.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Rowley SA, O'Callaghan FJ, Osborne JP. Ophthalmic manifestations of tuberous sclerosis: a population based study. British Journal of Ophthalmology. 2001;85(4):420-3.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Aronow ME, Nakagawa JA, Gupta A, Traboulsi EI, Singh AD. Tuberous Sclerosis Complex: Genotype/Phenotype Correlation of Retinal Findings. Ophthalmology. 2012;119(9):1917-23.
- ↑ Ulbright TM, Fulling KH, Helveston EM. Astrocytic tumors of the retina. Differentiation of sporadic tumors from phakomatosis-associated tumors. Arch Pathol Lab Med. 1984;108(2):160-3.
- ↑ Bec P, Mathis A, Adam P, Maillard P, Alberge Y. Retinitis pigmentosa associated with astrocytic hamartomas of the optic disc. Ophthalmologica. 1984;189(3):135-8.
- ↑ Iovino C, Casini G, Peiretti E. Bilateral noncalcified astrocytic hamartomas in retinitis pigmentosa: Multimodal imaging evaluation over 8 years of follow-up. Eur J Ophthalmol. 2019;29(5):NP18-NP21.
- ↑ Jump up to: 9.0 9.1 9.2 Kinori M, Moroz I, Rotenstreich Y, Yonath H, Fabian ID, Vishnevskia-Dai V. Bilateral presumed astrocytic hamartomas in a patient with retinitis pigmentosa. Clin Ophthalmol. 2011;5:1663-5.
- ↑ Awan KJ. Presumed glial retinal hamartomas in Usher's syndrome. Can J Ophthalmol. 1976;11(3):258-60.
- ↑ Bini A, Sodi A, Passerini I, Menchini U, Torricelli F. Retinal astrocytic hamartoma and Stargardt's disease: unusual association in a patient with ABCR mutation without phacomatosis. Clinical & Experimental Ophthalmology. 2007;35(8):777-9.
- ↑ Kiratli H, Turkcuoglu P, Bilgic S. Gyrate atrophy associated with astrocytic hamartoma of the optic disc. Retina. 2004;24(6):976-7.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 Mennel S, Meyer CH, Eggarter F, Peter S. Autofluorescence and Angiographic Findings of Retinal Astrocytic Hamartomas in Tuberous Sclerosis. Ophthalmologica. 2005;219(6):350-6.
- ↑ Gündüz K, Eagle RC, Jr., Shields CL, Shields JA, Augsburger JJ. Invasive giant cell astrocytoma of the retina in a patient with tuberous sclerosis. Ophthalmology. 1999;106(3):639-42.
- ↑ Jump up to: 15.0 15.1 15.2 Northrup H, Krueger DA, Northrup H, Krueger DA, Roberds S, Smith K, et al. Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatric Neurology. 2013;49(4):243-54.
- ↑ Shields CL. Retinal Pigment Epithelial Depigmented Lesions Associated With Tuberous Sclerosis Complex. Archives of Ophthalmology. 2012;130(3):387.
- ↑ Peng MY, Agarwal A, McDonald HR. Multimodal Imaging Findings in a Retinal Astrocytic Hamartoma. Ophthalmology. 2021;128(1):99.
- ↑ Kato A, Obana A, Gohto Y, Seto T, Sasano H. Optic coherence tomography appearances of retinal astrocytic hamartoma and systemic features in tuberous sclerosis of Japanese patients. Eur J Ophthalmol. 2019;29(3):330-7.
- ↑ Zhang C, Xu K, Long Q, Yang Z, Dai R, Du H, et al. Clinical features and optical coherence tomography findings of retinal astrocytic hamartomas in Chinese patients with tuberous sclerosis complex. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):887-92.
- ↑ Jump up to: 20.0 20.1 20.2 Querques G, Kerrate H, Leveziel N, Coscas G, Soubrane G, Souied EH. Intravitreal ranibizumab for choroidal neovascularization associated with retinal astrocytic hamartoma. Eur J Ophthalmol. 2010;20(4):789-91.
- ↑ Pichi F, Massaro D, Serafino M, Carrai P, Giuliari GP, Shields CL, et al. Retinal Astrocytic Hamartoma: Optical Coherence Tomography Classification and Correlation With Tuberous Sclerosis Complex. Retina. 2016;36(6):1199-208.
- ↑ Jump up to: 22.0 22.1 22.2 Gündüz AK, Mirzayev I, Kasimoglu R, Özalp Ateş FS. Swept-source optical coherence tomography angiography findings in choroidal and retinal tumors. Eye. 2021;35(1):4-16.
- ↑ Toledo JJ, Asencio M, Garcia JR, Morales LA, Tomkinson C, Cajigal C. OCT Angiography: Imaging of Choroidal and Retinal Tumors. Ophthalmol Retina. 2018;2(6):613-22.
- ↑ Jump up to: 24.0 24.1 Koak N, Saatci AO, Kaynak S, Ergin MH, Ingil GC. Indocyanine green angiography of retinal astrocytomas associated with tuberous sclerosis. Korean Journal of Ophthalmology. 2003;17(2):145.
- ↑ Shields CL, Schoenberg E, Kocher K, Shukla SY, Kaliki S, Shields JA. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases: results based on age at presentation. Ophthalmology. 2013;120(2):311-6.
- ↑ Shields JA, Shields CL, Ehya H, Buckley E, De Potter P. Atypical retinal astrocytic hamartoma diagnosed by fine-needle biopsy. Ophthalmology. 1996;103(6):949-52.
- ↑ Schwartz SG, Harbour JW. Spectral-Domain Optical Coherence Tomography of Presumed Solitary Circumscribed Retinal Astrocytic Proliferation Versus Astrocytic Hamartoma. Ophthalmic Surg Lasers Imaging Retina. 2015;46(5):586-8.
- ↑ Shields JA, Bianciotto CG, Kivela T, Shields CL. Presumed solitary circumscribed retinal astrocytic proliferation: the 2010 Jonathan W. Wirtschafter Lecture. Arch Ophthalmol. 2011;129(9):1189-94.
- ↑ Lois N, Shields CL, Shields JA, Eagle RC, Jr., De Potter P. Cavitary melanoma of the ciliary body. A study of eight cases. Ophthalmology. 1998;105(6):1091-8.
- ↑ Cennamo G, Romano MR, Breve MA, Velotti N, Reibaldi M, De Crecchio G, et al. Evaluation of choroidal tumors with optical coherence tomography: enhanced depth imaging and OCT-angiography features. Eye. 2017;31(6):906-15.
- ↑ Jump up to: 31.0 31.1 Grenga PL, Sagoo MS, Malagola R. Recurrent vitreous haemorrhage from sporadic retinal astrocytic hamartoma. Eye. 2007;21(5):682-4.
- ↑ Jump up to: 32.0 32.1 Vrabec TR, Augsburger JJ. Exudative retinal detachment due to small noncalcified retinal astrocytic hamartoma. American Journal of Ophthalmology. 2003;136(5):952-4.
- ↑ Arora AK, Fabian ID, Cohen VML. Subretinal Hemorrhage Associated with Astrocytic Hamartoma. Ophthalmology. 2017;124(4):571.
- ↑ Shields JA, Eagle RC, Jr., Shields CL, Marr BP. Aggressive retinal astrocytomas in four patients with tuberous sclerosis complex. Trans Am Ophthalmol Soc. 2004;102:139-47; discussion 47-8.
- ↑ Curatolo P, Moavero R. mTOR Inhibitors in Tuberous Sclerosis Complex. 2012;10(4):404-15.
- ↑ Marciano S, Mutolo MG, Siracusano M, Moavero R, Curatolo P, Emberti Gialloreti L. Everolimus for Retinal Astrocytic Hamartomas in Tuberous Sclerosis Complex. Ophthalmol Retina. 2018;2(3):257-60.
- ↑ Zhang ZQ, Shen C, Long Q, Yang ZK, Dai RP, Wang J, et al. Sirolimus for Retinal Astrocytic Hamartoma Associated with Tuberous Sclerosis Complex. Ophthalmology. 2015;122(9):1947-9.
- ↑ Nallasamy N, Seider MI, Gururangan S, Mruthyunjaya P. Everolimus to treat aggressive retinal astrocytic hamartoma in tuberous sclerosis complex. J AAPOS. 2017;21(4):328-31.
- ↑ Nakayama M, Keino H, Hirakata A, Okada AA, Terado Y. Exudative retinal astrocytic hamartoma diagnosed and treated with pars plana vitrectomy and intravitreal bevacizumab. Eye. 2012;26(9):1272-3.

