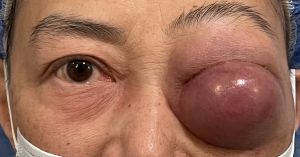
Orbital Plasmacytoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Orbital plasmacytomas are rare plasma cell neoplasms that can be either primary (isolated or solitary) or secondary (metastatic). Although rare, primary orbital solitary plasmacytomas (SP) can originate from bone (most commonly) or from soft tissue. Secondary plasmacytomas are more common, aggressive and are often manifestations manifestations of multiple myeloma. [1]
Etiology
Plasma cells are differentiated B-cells specialized to produce large amounts of immunoglobulin. Plasmacytomas are neoplasias due to a plasma cell proliferative disorder of monoclonal immunoglobulin chains. Solitary plasmacytomas as classified as solitary bone plasmacytomas (SBP) or solitary extramedullary plasmacytoma (SEP) depending on whether the sit involved is the bone versus the soft tissue.[2] Solitary bone plasmacytomas most frequently arise as bony lesions in the axial skeleton. Extramedullary plasmacytomas (EMP) are less common and can be found in soft tissues.[3] Both diseases represent a localized process with absence of systemic disease and/or end organ damage. It is though that these lesions fall somewhere in the spectrum between Monoclonal Gammopathy of Undetermined Significance (MGUS) and Multiple Myeloma (MM) as patients can go on to develop systemic myeloma.[3] A diagnosis of EMP or SPB can only be made after a systemic work-up, including lymph node assessment, skeletal survey, and bone marrow biopsy is negative. EMPs, SPBs, and secondary plasmacytomas related to multiple myeloma can all occur in the orbit.
Risk Factors
Increased age especially males, people of African and middle eastern descent, previous exposure to chemical and irradiation, those with a history of monoclonal gammopathy of undetermined ethology and overt multiple myeloma are at increased risk for development of orbital plasmacytomas.
General Pathology
The histopathologic appearance of plasmacytomas demonstrates a dense monomorphous plasma cell infiltrate, with a heterogenous cell morphology with mature myeloma being indistinguishable from normal cells. Some cells may be binucleated or multinucleated and nuclei may show irregular margins, demonstrate infolding and lobulation, or show a diffuse chromatin pattern. The surrounding stroma is typically scant, with delicate thin-walled vessels that have a tendency to bleed.[4] Immunohistochemical staining typically shows monoclonality with CD138 positivity. Abnormal expression of CD56 or CD38 can also be seen.[5]
Primary and well-differentiated secondary plasmacytomas cannot be differentiated histologically and must be distinguished clinically with appropriate diagnostic evaluation for systemic involvement.[4] The most common multiple myeloma subtype is IgG, followed by IgA. Similarly, IgG and IgA subtypes are most commonly found in patients with orbital involvement.[6][7]
Primary Prevention
Adequate treatment of multiple myeloma may prevent the occurrence of formation of secondary orbital plasmacytomas.
Diagnosis
History
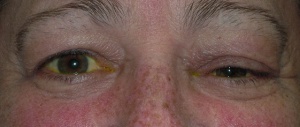
Patients with orbital plasmacytomas most commonly present with unilateral proptosis.[6] Bilateral presentations are less common but can be seen.[8] Ptosis, eyelid swelling, and doubled or blurred vision may be present. Uncommon presentations may include periorbital ecchymosis, orbital hemorrhage, fungating eyelid lesions, orbital abscess, orbital cellulitis, or Tolosa Hunt syndrome.[9] [10] [11] [12] [13] [14][15] Patients may also have systemic manifestations of the multiple myeloma, including: fatigue, infections, pathologic fractures, or bone pain.[4] In the largest study to date published by Thuro et al, over half of patients presenting with and orbital plasmacytoma had a known diagnosis of multiple myeloma.[8] The typical age of presentation of multiple myeloma is between 40-70 years old. Similarly, the average age of presentation in patients with orbital disease is 56.5 years old, with an equal distribution between men and women.
Physical examination
Patients with primary or secondary orbital plasmacytomas can present similarly with signs that are non-specific and may be seen in several orbital disease process. Thus, all patients should have a complete ophthalmic examination. External exam may reveal many of the common physical exam findings including proptosis, ptosis, or eyelid changes. In these circumstances, exophthalmometry and marginal reflex distance should be recorded. Baseline photographs should be taken to follow progress and to monitor for progression. Best-corrected visual acuity should be recorded in addition to the presence of motility deficits. Lesions can compromise the optic nerve so a careful pupillary exam looking for the presence of a relative afferent pupillary defect must be performed. If there is concern for compression of the optic nerve, the presence of a relative afferent pupillary defect should be checked, and color vision testing and automated visual field testing should be considered. In patients with secondary orbital plasmacytomas, pathologic changes can be present in both the anterior and posterior segment, so a full ocular exam, including a dilated fundus exam should be performed.
Signs
Orbital plasmacytomas may present with signs that are non-specific and may be seen in numerous orbital disease process, including ptosis, proptosis, diplopia, or conjunctival congestion. Blurred vision, diplopia and increased tearing have also been reported.[4] In patients with secondary plasmacytomas from multiple myeloma ocular findings may include: exudative macular detachment, choroidal mass, crystal or copper deposition in the cornea,[16] ciliary body cysts, retinal hemorrhages, and cotton-wool spots.[6][17]
Symptoms
Patients present with proptosis, ptosis, eyelid swelling, tearing, diplopia or decreased vision. Pain can be present, but is less common.
Diagnostic procedures
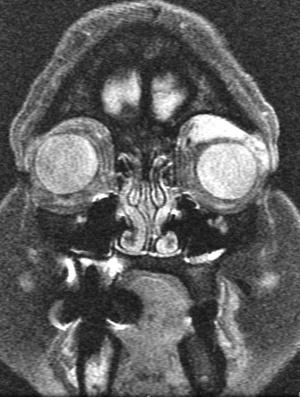
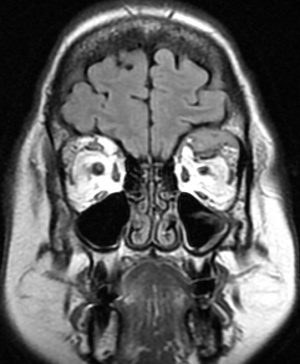
Diagnosis of orbital plasmacytoma can only be made with histopathologic examination of a biopsy of the lesion although classic radiologic features in a patient with or without known multiple myeloma may be suggestive as well. Plasmacytomas are found in the posterior orbit 69% of the time and are extraconal 90% of the time.[6] Most commonly, patients have a superotemporal bony lesion involving the sphenoid or frontal bone with orbital extension causing a mass effect.[6][8] Other radiographic appearances include a lesion infiltrating across anatomical planes including those originating from the sinuses and those emanating from the orbital floor. Bony destruction can be found with these lesions. Additional laboratory tests should be considered in all patients once a diagnosis of plasmacytoma has been established, especially patients without a known history of Multiple Myeloma as a plasmacytoma can be an initial presenting sign.[8] These include tests for anemia, hypoproteinemia, Bence Jones proteinuria, and abnormal proteinemia. Further testing may find hypercalcemia (due to bone destruction), hyperuricemia (resulting from high cell turn over), and elevated ESR. Hypergammaglobulinemia is present in 85% of cases of multiple myeloma.
Differential diagnosis
Differential diagnosis for orbital plasmacytomas include other lymphoproliferative disorders, lacrimal gland tumors, mesenchymal tumors. and inflammatory orbital 'pseudotumors'. The histologic differential diagnosis includes idiopathic lymphoproliferative disease, undifferentiated sarcoma, amelanotic melanoma, and reticulum cell sarcoma.[4]
Management
General treatment
External beam radiation is the primary treatment for primary localised plasmacytomas. Sometimes surgical excision and/or debulking or chemotherapy may be necessary. Rarely, EMPs are unresponsive to radiation and chemotherapy, and may require radical surgery like exenteration. Secondary plasmacytomas can be treated with a radiation and/or surgical excision. In conjunction with an oncologist,
underlying multiple myeloma should be treated with chemotherapy and/or bone marrow transplant. Three-drug chemotherapy, described below, is the gold standard for initial treatment (induction chemotherapy), which may precede or occur in conjunction with autologous hematopoietic stem cell transplant. When multiple myeloma recurs or persists, chimeric antigen receptor T-cell therapy (CAR-T therapy) may be considered.
Medical therapy
When systemic disease / multiple myeloma is present, three-drug chemotherapy comprised of a proteasome inhibitor, immunomodulatory drug, and steroid is utilized (Table 1). A common example is the bortezomib, lenalidomide, and dexamethasone protocol. Another variation consists of Daratumumab, a monoclonal antibody to CD38, in lieu of a proteasome inhibitor. Chemotherapy should be administered by an oncologist for patients with multiple myeloma. Newer agents for refractory systemic disease include Selexinor, a Selective Inhibitor of Nuclear Export(SINE), Elotuzumab that targets molecule SLAMF7, Daratumumab, a CD 38 target agent, Isaluxumab, Ixazomib, Panobinostat, etc. On the horizon are several new therapeutic options that include Antibody Drug Conjugates (ADCs), T-cell engagers and CAR T-Cell therapies, which are making big inroads into various hematological malignancies including lymphomas.
Table 1
| Three-Drug Regimen | Mechanism of Action | Overall Response Rate | Complete Response, VGPR, PFS | Unique features |
| DRd (daratumumab, lenalidomide, dexamethasone) | Monoclonal antibody to CD 38 + immunomodulator + steroid | 66% | CR = 51% | |
| VRd (bortezomib, lenalidomide, dexamethasone) | Proteasome inhibitor + immunomodulator + steroid | 82% | CR = 16% | |
| VTd (bortezomib, thalidomide, dexamethasone) | Proteasome inhibitor + immunomodulator + steroid | 92% | VGPR = 66% | Used in acute renal failure, second line to VRd |
| VCd aka CyBorD (bortezomib, cyclophosphamide, dexamethasone) | Proteasome inhibitor + DNA crosslinker + steroid | 82% | VGPR = 41% | Used in acute renal failure, inferior to VTd, high toxicity |
| KRd (carfilzomib, lenalidomide, dexamethasone) | Proteasome inhibitor + immunomodulator + steroid | 86% | PFS = 35 months | High rates of end-organ damage |
| IRd (ixazomib, lenalidomide, dexamethasone) | Proteasome inhibitor + immunomodulator + steroid | 73% | PFS = 17 months | Not as effective as VRd or DRd |
Table 1: commonly used three-drug regimens for induction chemotherapy in multiple myeloma
CAR-T Therapy
For refractory or resistant cases of multiple myeloma, chimeric antigen receptor T-cell therapy (CAR-T) may be appropriate. A patient's T cell's are biologically engineered to express chimeric antigen receptors (CARs) which latch onto B-cell maturation antigen on tumor cells. This leads to the release of pro-inflammatory cytokines, causing cytolysis and resultant tumor cell death. CAR-T therapy has been demonstrated in several clinical trials to have an overall response rate between 73 and 81%, and average progression-free survival of 8-12 months. [18]
Autologous Hematopoietic Stem Cell Transplant
In certain cases, autologous hematopoietic stem cell transplantation (AHSCT) may also be indicated. Eligibility is determined at time of diagnosis and is based on several criteria including age < 75 years, absence of liver cirrhosis and class III or IV heart failure, and overall functional status.[19] Careful consideration should also be taken in patients with a history of renal impairment. AHSCT may be part of initial treatment, or deferred until first relapse as early AHSCT has not translated into an overall survival benefit.[20] Typically, patients are treated with 3-4 cycles of induction therapy with bortezomib, lenalidomide, dexmethasone (VRd) prior to stem cell harvest.[20] A 2020 review of 4300 patients found that the current 5-year survival is 68%, which reflects an upward trend since 1997. Progression-free survival is 73% at 3 years. [21]
Surgery
Radiation therapy is the mainstay of treatment, but surgical debulking or complete excision may be an option for some patients
Surgical follow up
Regular surgical follow-up is recommended. Additionally, patients should be followed at regular intervals for surveillance of tumor recurrence.
Complications
Complications associated with surgical removal are typical for any orbital surgery including retrobulbar hemorrhage, diplopia, ptosis, strabismus, decreased vision, blindness, or incomplete tumor excision.
Prognosis
Although the prognosis for patients with multiple myeloma has improved over the years, mostly due to improvements in chemotherapeutic agents, it still remains poor, ranging from 29 to 62 months and may depend on presence of certain biomarkers.[22] [23] [24] The current 10 year survival is only about 18%.[7] In patients presenting with plasmacytomas, prognosis is generally better for primary solitary plasmacytomas than for secondary plasmacytomas(multiple myeloma). In fact, the mean survival for patients with EMPs, from any location, has been reported to be 8.3 years.[4] On the contrary, EMPs of the orbit may actually portend a poor prognosis, having a reported survival of only 28 months.[6] In patients with primary plasmacytomas, up to 75% will go on to develop systemic multiple myeloma.[6]
References
- ↑ Adkins JW, Shields JA, Shields CL, Eagle RC, Flanagan JC, Campanella PC. Plasmacytoma of the eye and orbit. Int Ophthalmol. 20(6):339-343.
- ↑ Pham A, Mahindra A. Solitary Plasmacytoma: a Review of Diagnosis and Management. Curr Hematol Malig Rep. 2019 Apr;14(2):63-69
- ↑ Jump up to: 3.0 3.1 Bhadauria M, Ranjan P, Mishra D. Primary orbital plasmacytoma mimicking lacrimal gland tumor. Orbit. 2014;33(4):305-307.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 De Smet M, Rootman J. Orbital manifestations of plasmacytic lymphoproliferations. Ophthalmology. 1987;94(8):995-1003.
- ↑ Fujino M. The histopathology of myeloma in the bone marrow. J Clin Exp Hematop. 2018;58(2):61-67. doi: 10.3960/jslrt.18014. PMID: 29998977; PMCID: PMC6413148
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Burkat CN, Van Buren JJ, Lucarelli MJ. Characteristics of orbital multiple myeloma: a case report and literature review. Surv Ophthalmol. 54(6):697-704.
- ↑ Jump up to: 7.0 7.1 Zloto O et al. Periocular Presentation of Solitary Plasmacytomas and Multiple Myeloma. Ophthalmic Plast Reconstr Surg. 2021 Jul 21. doi: 10.1097/IOP.0000000000002023. PMID: 34293789.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Thuro, Bradley A. M.D.*; Sagiv, Oded M.D.*; Shinder, Roman M.D.†; Debnam, J. Matthew M.D.‡; Ozgur, Omar M.D.*; Ng, John D. M.D., F.A.C.S.§; Rootman, Daniel M.D.‖; Thomas, Sheeba K. M.D.¶; Esmaeli, Bita M.D., F.A.C.S.* Clinical Presentation and Anatomical Location of Orbital Plasmacytomas, Ophthalmic Plastic and Reconstructive Surgery: May/June 2018 - Volume 34 - Issue 3 - p 258-261 doi: 10.1097/IOP.0000000000000939
- ↑ Goldstein DA, Schteingart MT, Birnbaum AD, Tessler HH. Bilateral eyelid ecchymoses and corneal crystals: an unusual presentation of multiple myeloma. Cornea. 2005;24(6):757-758.
- ↑ Kelly SP, Lloyd IC, Anderson H, Joyce PW, Pace-Balzan A. Solitary extramedullary plasmacytoma of the maxillary antrum and orbit presenting as acute bacterial orbital cellulitis. Br J Ophthalmol. 1991;75(7):438-439.
- ↑ Rappaport K, Liesegang TJ, Menke DH, Czervionke LF. Plasmacytoma manifesting as recurrent cellulitis and hematic cyst of the orbit. Am J Ophthalmol. 1996;122(4):595-597.
- ↑ Almousa R, Shuen C, Sundar G. Acute orbital hemorrhage as a presentation of a lytic bony lesion. Craniomaxillofac Trauma Reconstr. 2011;4(4):189-192.
- ↑ Sen S, Kashyap S, Betharia S. Primary orbital plasmacytoma: A case report. Orbit. 2003;22(4):317-319.
- ↑ Ko S, Huang S-Y, Liu C-Y. Extramedullary plasmacytoma masquerading as Tolosa-Hunt syndrome: a case report. BMJ Case Rep. 2009;2009.
- ↑ Russell DJ, Seiff SR. Orbital Plasmacytoma Mimicking an Orbital Abscess. Ophthalmic Plast Reconstr Surg. 2017 Mar/Apr;33(2):e32-e33. doi: 10.1097/IOP.0000000000000685. PMID: 27046038.
- ↑ Silkiss RZ, Pomerleau D, Sorenson A, Vastine D, Crawford JB. Corneal cupremia in multiple myeloma: a clinicopathologic correlation. Arch Ophthalmol. 2008;126(7):1005-1006.
- ↑ Choi JH, Kim JH, Jeong JG. Orbital and Choroidal Plasmacytoma in Recurrent Multiple Myeloma: A Case Report. Korean J Ophthalmol. 2021;35(4):335-336
- ↑ Teoh, P.J., Chng, W.J. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 11, 84 (2021). https://doi.org/10.1038/s41408-021-00469-5
- ↑ Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019 Apr 8;9(4):44. doi: 10.1038/s41408-019-0205-9. PMID: 30962422.
- ↑ Jump up to: 20.0 20.1 Rajkumar SV. Multiple myeloma: Every year a new standard? Hematol Oncol. 2019;37 Suppl 1(Suppl 1):62-65. doi:10.1002/hon.2586
- ↑ Nishamura et al. Long-term outcomes after autologous stem cell transplantation for multiple myeloma. Blood advances. 2020; 4(2): 422-431.
- ↑ Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-3420.
- ↑ Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516-2520.
- ↑ João C, Costa C, Coelho I, Vergueiro MJ, Ferreira M, da Silva MG. Long-term survival in multiple myeloma. Clin case reports. 2014;2(5):173-179.