Ophthalmologic Manifestations of Spinocerebellar Ataxia
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Spinocerebellar ataxia (SCA) describes a group of hereditary neurodegenerative disorders characterized by a slowly progressive ataxia. Early symptoms include difficulty with gait and balance and as the disease progresses may include poor coordination of hand movements, eye movements, and speech. [1][2] These are secondary to degeneration of the cerebellum or its associated pathways. [3] While there are several inherited, sporadic, and acquired causes of cerebellar ataxia, the term SCA specifically refers to the autosomal dominant cerebellar ataxias (ADCA). Currently there are 40 identified SCAs, numbered SCA1 through SCA40, and that list continues to grow. [4]
The prevalence of SCA in the general population is estimated be 1 to 5 per 100,000 people. [5] SCA3, also known as Machado-Joseph Disease, is the most common SCA worldwide. SCAs 1, 2, 6, 7, and 8 account for the next most common forms of SCA, each accounting for at least 2% of SCAs. The other forms of SCA are considered very rare. The onset of symptoms is typically between 30 and 50 years of age but has been reported as early as childhood and as late as 70 years of age. [2] The etiology and presentation of each SCA is unique. Ophthalmologic manifestations vary depending on the type of SCA involved. Symptoms may include ophthalmoplegia, optic atrophy, pigmentary retinal degeneration, nystagmus, and/or saccadic changes. [6][7]
Etiology
SCA is a group of hereditary ataxias showing an autosomal dominant inheritance pattern. While the etiology of each SCA is unique and some are still unknown, many are caused by an expansion of Cytosine-Adenine-Guanine (CAG) nucleotide repeats. These trinucleotide repeats are transcribed and translated into polyglutamine protein products that are thought to produce a toxic gain of function. These mutations are dynamic and the number of repeats increases from generation to generation through a genetic phenomenon known as anticipation. As these repeats continue to grow in successive generations, they cause an earlier onset and more severe form of the disease. [2] The penetrance SCA is very high, meaning people who inherit a repeat expansion will develop symptoms of SCA at some point in their lifetime.
Risk Factors
CAG repeat length affects the age of onset and severity of disease. [8]
Pathophysiology
Multiple neurodegenerative diseases are caused by expansion of CAG nucleotide repeats. Except for the encoded polyglutamine repeats, the affected proteins found within different SCAs do not share common sequences or domains. For example, SCA6 affects the α1A-subunit of a P/Q-type calcium channel and SCA17 affects a TATA-box binding protein on a different chromosome. Although it is theoretically possible that expanded repeat at the level of mRNA causes toxicity, evidence suggests that the encoded protein with its enlarged polyglutamine tract causes the pathogenesis. [9] When the threshold of approximately 35–40 triplet repeat units is exceeded, the glutamine repeat is susceptible to abnormal conformations and aggregation while interfering with other neuronal proteins. [9] Ordway et al demonstrated that expanded polyglutamine repeats inserted into a foreign protein of transgenic mice produced a neurological phenotype and neuropathological features comparable to SCA. [10] Neuronal inclusions composed of expanded polyglutamine tracts are a pathological hallmark of SCA. [11] The polyglutamine disorders are unique in that only a select subset of neurons is vulnerable although the pathogenic proteins are widely expressed throughout the nervous system.
Primary Prevention
Prenatal testing and testing for at-risk relatives is currently an option for several types of SCA. However, some genetic causes of SCA remain unknown and testing may not provide a definitive diagnosis.[6]
Diagnosis
Signs & Symptoms
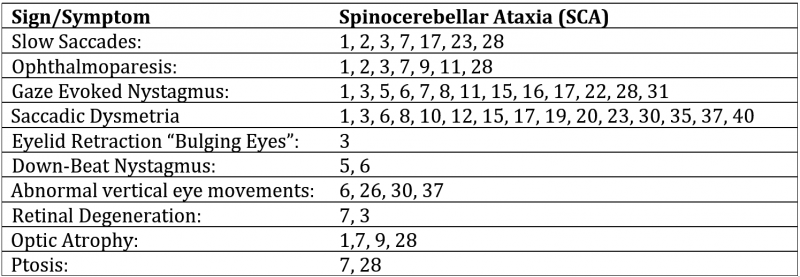
Differentiating the various types of SCA on clinical manifestations alone is difficult, as all exhibit problems with movement that worsen over time. While no individual sign is pathognomonic, some ophthalmologic signs are far more prevalent in specific SCAs. These combined with other clinical manifestations can help narrow down the diagnosis and direct genetic testing. As stated above, a few subtypes of SCA account for the vast majority of reported cases and their more unique ophthalmologic features are highlighted below:
- SCA1: Saccadic dysmetria. While many types of SCA may present with saccadic dysmetria, a disproportionate number of SCA1 present with hypermetric saccades. [4] There are some reports of optic atrophy with this in approximately 33% of patients with vision loss that progressively worsens over time. Central/cecocentral scotoma and dyschromatopsia may be present. [12]
- SCA2: Prominent slow saccades are found early in the disease, while other types of SCA may present with slow saccades late in disease progression. There is little to no gaze-evoked nystagmus or dysmetric saccades present, separating it from other more common forms of SCA. [4][13]
- SCA3: “Bulging eyelids” secondary to eyelid retraction with ophthalmoparesis is highly correlated with SCA3 but is not pathognomonic. [14]. There are case reports that have found both gaze-evoked nystagmus and ophthalmoparesis along with pigmentary retinopathy. [15]
- SCA6: Downbeat nystagmus. [4]
- SCA7: Retinal degeneration, with early blue-yellow color blindness. Pigmentary retinopathy. [4]
The clinical signs of SCA are elicited through a neurological exam, including poorly coordinated gait, dysarthria, and the abnormal ophthalmologic manifestations outlined above. Acquired causes of ataxia must be ruled out (see Differential diagnosis below) as there may be a specific treatment available to those patients. Finally, a positive family history of ataxia, detection of gene mutation consistent with SCA via genetic testing, or a constellation of symptoms consistent with a specific SCA phenotype is sufficient for diagnosis. Ancillary testing, such as computed tomography or magnetic resonance imaging, may be beneficial in the minority of patients who have a subtype of SCA for which commercial genetic testing is not available. [1]
Differential diagnosis
Ruling out non-hereditary causes of ataxia is important, because unlike SCA, many of these are treatable. Causes of ataxia include intoxication (alcohol), cerebellar masses/infarcts, cerebellar degeneration (alcoholism), vitamin deficiencies, multiple sclerosis, vascular disease, tumors, and paraneoplastic diseases of the breast, lung, ovary, uterus, or cervix, and multiple system atrophy.[1]
Management
At this time there is no curative treatment for SCA. Current treatment strategies are symptomatic and palliative, as many patients report tremors, stiffness, muscle spasms, and sleep disorders in addition to their ataxia. Trials have included nicotinic receptor agonists (varenicline), 5-HT1A agonists, enhancing GABAergic transmission, cholinergic enhancement, channel stabilizers, IGF-1, and others with mixed results. [16] Physical therapy and devices including canes, walkers, and wheelchairs may assist in mobility.
Prognosis
The long-term outlook for people with SCA is often dependent on the type of SCA and the number of trinucleotide repeats. Most information on the prognosis of SCA is based on SCA1, SCA2, SCA3, and SCA6. These patients are usually wheelchair bound 15-20 years after the onset of symptoms. [6][8]
Additional Resources
- Turbert D, Mendoza O. What is Color Blindness?. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/what-is-color-blindness. Accessed February 27, 2023.
References
- ↑ Jump up to: 1.0 1.1 1.2 Jayadev S, Bird TD. Hereditary ataxias: overview. Genetics in Medicine. 2013 Sep;15(9):673.
- ↑ Jump up to: 2.0 2.1 2.2 Schöls L. Autosomal dominant spinocerebellar ataxias. Orphanet Encyclopedia. 2003.
- ↑ Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. The Lancet Neurology. 2004 May 1;3(5):291-304.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Sun YM, Lu C, Wu ZY. Spinocerebellar ataxia: relationship between phenotype and genotype–a review. Clinical genetics. 2016 Oct 1;90(4):305-14.
- ↑ Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174-83.
- ↑ Jump up to: 6.0 6.1 6.2 Paulson HL. The spinocerebellar ataxias. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2009 Sep;29(3):227.
- ↑ Rossi M, Perez‐Lloret S, Doldan L, Cerquetti D, Balej J, Millar Vernetti P, Hawkes M, Cammarota A, Merello M. Autosomal dominant cerebellar ataxias: a systematic review of clinical features. European journal of neurology. 2014 Apr 1;21(4):607-15.
- ↑ Jump up to: 8.0 8.1 Klockgether T, Lüdtke R, Kramer B, Abele M, Bürk K, Schöls L, Riess O, Laccone F, Boesch S, Lopes-Cendes I, Brice A. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain: a journal of neurology. 1998 Apr 1;121(4):589-600.
- ↑ Jump up to: 9.0 9.1 Rüb U, Schöls L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K, Korf HW, Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Progress in neurobiology. 2013 May 1;104:38-66.
- ↑ Ordway JM, Tallaksen-Greene S, Gutekunst CA, Bernstein EM, Cearley JA, Wiener HW, Dure IV LS, Lindsey R, Hersch SM, Jope RS, Albin RL. Ectopically expressed CAG repeats cause intranuclear inclusions and a progressive late onset neurological phenotype in the mouse. Cell. 1997 Dec 12;91(6):753-63.
- ↑ Paulson HL, Shakkottai VG, Clark HB, Orr HT. Polyglutamine spinocerebellar ataxias—from genes to potential treatments. Nature Reviews Neuroscience. 2017 Oct;18(10):613.
- ↑ Abe T, Abe K, Aoki M, Itoyama Y, Tamai M. Ocular changes in patients with spinocerebellar degeneration and repeated trinucleotide expansion of spinocerebellar ataxia type 1 gene. Arch Ophthalmol. 1997;115(2):231-6.
- ↑ Moscovich M, Okun MS, Favilla C, Figueroa KP, Pulst SM, Perlman S, Wilmot G, Gomez C, Schmahmann J, Paulson H, Shakkottai V. Clinical evaluation of eye movements in spinocerebellar ataxias: a prospective multicenter study. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2015 Mar;35(1):16.
- ↑ Pedroso JL, Souza PV, Pinto WB, Albuquerque MV, Barsottini OG. Eyelid retraction is not a pathognomonic sign of Machado-Joseph disease in the context of spinocerebellar ataxias. Arquivos de neuro-psiquiatria. 2014 Apr;72(4):326-7.
- ↑ Isashiki Y, Kii Y, Ohba N, Nakagawa M. Retinopathy associated with Machado--Joseph disease (spinocerebellar ataxia 3) with CAG trinucleotide repeat expansion. Am J Ophthalmol. 2001;131(6):808-10.
- ↑ Sarva H, Shanker VL. Treatment options in degenerative cerebellar ataxia: a systematic review. Movement Disorders Clinical Practice. 2014 Dec 1;1(4):291-8.