Ocular Manifestations of Osteogenesis Imperfecta
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Osteogenesis Imperfecta (Ocular Manifestations)
- ICD 10: Q78.0: Osteogenesis Imperfecta
Disease
Osteogenesis imperfecta (OI) is the most commonly inherited systemic connective tissue disease with the most significant manifestation presenting in the bone. While the earliest finding of osteogenesis imperfecta is from a partially mummified infant skeleton from ancient Egypt, it was first studied by Olof Jakob Ekman in 1788 and described as a “brittle bone disease”. Subsequently, in 1833, Jean Lobstein described osteogenesis imperfecta type I as Lobstein’s disease, and in the 1850s, Willem Vrolik described osteogenesis type II in what is currently known as Vrolik’s disease. [1][2]
Osteogenesis imperfecta is most often caused by mutations in the COL1A1 and COL1A2 genes that encode type I procollagen. Additionally, CRTAP, LEPRE1, and P3H1 gene mutations have also been linked to this disease. There are four major types of Osteogenesis Imperfecta with variable disease presentation and overlapping characteristics. The spectrum may range from almost asymptomatic patients with mild impact on stature, fractures, dentition and life span to patients with severe skeletal deformations, hearing loss, very short stature, dentinogenesis imperfecta and even perinatal mortality. While the skeletal manifestations are more predominant, OI can also present with ocular complications such as blue sclera, hyperopic or myopic eyes, retinal detachments, decreased corneal rigidity, and glaucoma. [1][3][2][4]
Epidemiology
In the United States, the prevalence of osteogenesis imperfecta is estimated to be 1 per 20,000 live births. While the disease is predominantly known to be autosomal dominant, inheritance is not always as clearly defined as de novo mutations may occur. In addition, mild forms of OI de novo mutations are typically more severe causing more than 60% of classic non-deforming or common variable OI with normal sclerae, and almost 100% of progressively deforming and perinatally lethal OI.
It is also important to note that mild forms of osteogenesis imperfecta may be underdiagnosed therefore actual prevalence may be higher. Prevalence worldwide is similar to the United States; however, there may be an increased risk of recessive forms of OI due to a higher likelihood of consanguinity. Osteogenesis imperfecta has no predisposition for a particular sex or race. [2]
Ocular Manifestations
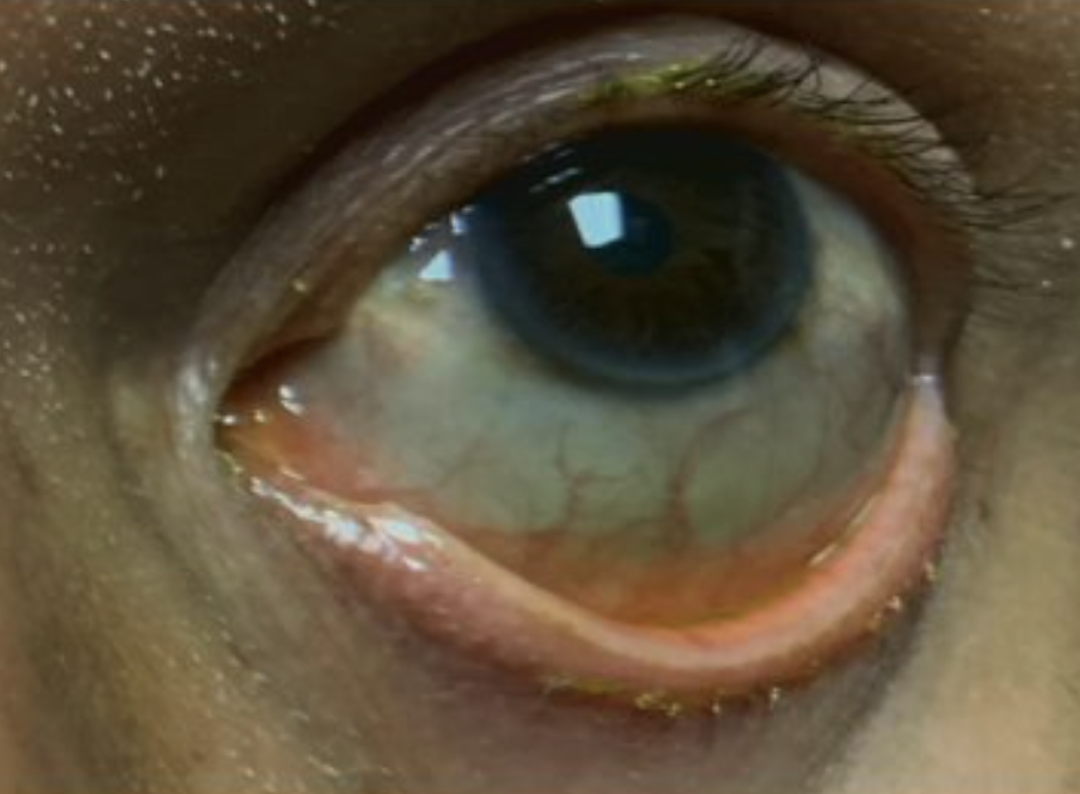
Osteogenesis imperfecta is predominantly known as a bone disease that can also have systemic manifestations. Blue sclera is the most commonly known ocular sign for osteogenesis imperfecta and it is caused by thin scleral collagen allowing the underlying darker choroid vasculature to be seen.
Patients with OI have shown a reduction in thickness of the corneal and scleral collagen fibers which can result in low ocular rigidity. OI patients may also have thin corneas, small corneal diameters, and keratoconus; however corneal ruptures are rare. Due to the significant impact of a corneal rupture or open globe, caution is always recommended in patients with connective tissue disorders, such as osteogenesis imperfecta.[5] A study by Ganesh et al. identified that children with OI had an increased risk for developing retinal hemorrhage and subdural trauma after minor injury.[6] Lagrou et al. further studied children with OI and found they had altered corneal structure and biomechanics. These children had decreased central corneal thickness, decreased corneal resistance factor and had a corneal compensation IOP significantly higher in OI cases than in the control group. Given this study, patients with OI should be monitored by an ophthalmologist for high IOP, development of glaucoma and other corneal pathologies. [7][8]
Type I collagen plays a significant role in the normal aqueous humor outflow and optic nerve function. It can be found in the trabecular meshwork (TM), uveoscleral pathway and the optic nerve. If there is a decrease in type I collagen in the TM, there can be an increase in the aqueous outflow resistance which can increase intraocular pressure. Similar effects would occur with decreased type I collagen in the uveoscleral pathway. As type I collagen is also found in the optic nerve head and lamina cribosa, these regions combined with possible elevated IOP can result in the optic nerve being more susceptible to damage. [9]
In the recent report by Feinstein et al. the OI population was surveyed from 2006-2013 to report any eye conditions. Of the patients that participated in the survey, the most common eye conditions were loss of vision and refractive errors. Other reported ocular conditions found in literature include glaucoma, cataract, keratoconus, macular degeneration, scleromalacia, floaters, retinal or vitreous hemorrhage, amblyopia, light sensitivity, retinal tears or detachments, poor eyelid closure, chorioretinitis, papilledema, and retinal thinning, congenital Bowman’s layer agenesis, choroidal neovascularization, optic neuropathies and atrophy. [10][11][12][8][13]
Ocular symptoms of OI can be life-changing and can cause permanent vision loss. Ophthalmologists should follow up with OI patients annually.
Genetics
Osteogenesis imperfecta is a polygenic disease that is most commonly inherited in an autosomal dominant pattern. More than 90 percent of all cases are caused by mutations in COL1A1 or COL1A2 gene. COL1A1 gene is located at chromosome 17 at position 21.33 (17q21.33), and COL1A2 is located at chromosome 7 at position 21.3 (7q21.3). The mildest forms of OI (Type I and Type IV) are typically inherited whereas severe infantile forms (such as Type II and Type III) occur due to de novo mutations. Less commonly, OI can also occur due to autosomal recessive pattern of inheritance. Some cases of OI type III can be caused by autosomal recessive inherited mutations in COL1A1 and COL1A2. Mutations can also be caused by CRTAP or PH3H1(LEPRE1) in an autosomal recessive pattern. [14]
Pathophysiology
The pathophysiology of osteogenesis imperfecta is primarily due to a dysfunction in collagen and is classified under connective tissue diseases.
The COL1A1 and COL1A2 gene contains instructions for creating type I collagen. Collagen is a necessary protein that provides strength to multiple tissues in the body. Type I collagen is specifically found in bone, cartilage, tendon, skin, and the many ocular tissues such as trabecular meshwork, optic nerve, sclera, and uveoscleral pathway. It is also the most abundant form of collagen in the body.
Collagen is created from procollagen, which is made of three chains. Triple stranded procollagen molecules are exported out of the cell and processed by multiple enzymes and cofactors to create the final mature collagen. Collagen strands then arrange themselves into long, thin fibrils with cross-linking to form a strong meshwork. Type I collagen is made up of two pro-α1(I) chains and one pro-α2(I) chain. COL1A1 mutations cause aberrant pro-α1(I) chains to form, whereas COL1A2 creates an aberrant pro-α2(I) chain. Therefore, poor procollagen formation yields an equally weak type I collagen to form throughout the body.
CRTAP gene (3p22.3) and P3H1/LEPRE1 (1p34.2) contain the instructions for making cartilage associated protein and enzyme prolyl-3-hydroxylase, respectively. Mutations in either gene cause problems in the normal protein folding, assembly, and secretion of the procollagen molecules resulting in weakened connective tissue and ultimate bone abnormalities, deformities, and short stature. CRTAP and P3H1 cause the rare, more severe types of OI – CRTAP gene mutations can cause type VII and P3H1 mutations cause type VIII. [14] [15] [16]
Types of Osteogenesis Imperfecta
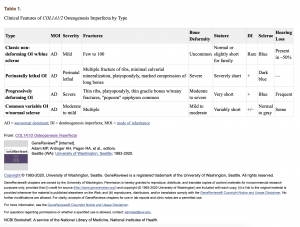
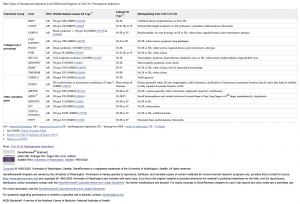
In 1979, Sillence described the four types of osteogenesis imperfecta caused by COL1A1/COL1A2. To this date, there continues to be significant variance on the classification system used for Osteogenesis Imperfecta. The Sillence classification discussed the four predominant types of OI (Type I, mild, common, with blue sclera; Type II, perinatal lethal form; Type III, severe and progressively deforming, with normal sclera; and Type IV, moderate severity with normal sclera) (See Table 1). The Osteogenesis Imperfecta Foundation discusses the six most common forms and indicates that 15 types of OI have been identified. [17] The NIH osteoporosis and related bone diseases national resource center discusses the eight most prevalent forms of OI.[18] Amongst all sources, the multitude of OI forms are consistently on a spectrum between the four types of OI originally discussed by the Sillence classification. There is currently no standard classification system being currently used and the identification of OI types are based on minor changes between characteristics and severity. [1][2]
Primary characteristics of the four types of COL1A1/COL1A2 Osteogenesis Imperfecta (COL1A1/2-OI)
- Classic non-deforming OI with blue sclerae (previously OI type I)
- Perinatally lethal OI (previously OI type II)
- Progressively deforming OI (previously OI type III)
- Common variable OI with normal sclerae (previously OI type IV)
Diagnosis
The diagnosis of osteogenesis imperfecta depends on consistent surveillance of the bones and systemic symptoms due to the variance in the presentation of the disease. Patient history, family history, genetic studies and radiological studies aid in the accurate diagnosis.
History
A thorough patient and family history is imperative to accurate diagnosis of osteogenesis imperfecta. Many cases of OI have an autosomal dominant inheritance pattern, therefore, a complete family history is a strong component to diagnosis.
Systemic Manifestations
- Blue/gray scleral hue
- Triangular facies
- Macrocephaly
- Progressive, post-pubertal hearing loss
- Defective dentition (or Dentinogenesis imperfecta)
- Barrel chest
- Scoliosis
- Limb deformities
- Fractures (with minimal or no trauma, after ruling out non-accidental trauma such as abuse; no known bone disorder)
- Joint laxity (ligamentous laxity)
- Short stature (shorter stature than anticipated based on the unaffected family)
- Bowed legs
- Constipation (may have been due to being wheelchair-bound)
- Cardiovascular disease (Ashournia et al. published a systemic review showing a higher prevalence of arterial and aortic dissection in OI patients)[2][21]
- Gross motor development retardation (due to joint hypermobility and increased limb deformity due to recurrent fractures)
Radiographic Features
- Fractures of varying ages and stages of healing. It predominantly affects the long bones but may also affect ribs and skull. If rib fractures are present, have a high index of suspicion for non-accidental trauma and rule it out prior to diagnosing as OI.
- "Codfish" vertebrae occur most commonly as adults and the appearance on the radiograph is due to spinal compression fractures.
- Wormian bones are defined as "sutural bones which are 6 mm by 4 mm (in diameter) or larger, in excess of ten in number, with a tendency to arrangement in a mosaic pattern.” Wormian bones are highly linked to OI but not pathognomonic.
- Protrusio acetabuli is a protruded acetabulum in the hip region. It is caused by a deep socket of the hip joint causing the acetabulum to bulge outwards
- Low bone mass (detected by DEXA scan) can vary depending on the type of OI. In addition, sample data for children is sparse thus reliability in usage for OI diagnosis is limited. DEXA scan can be used to monitor bone density over time in a single patient to deduce the progression of the disease.
Differential Diagnosis
- Perinatal hypophosphatasia
- Thanatophoric dysplasia
- Ehlers-Danlos
- Achondrogenesis type 1B
- Campomelic dysplasia
- Non-accidental trauma
- Osteoporosis pseudoglioma syndrome
Primary Prevention
Avoidance of dangerous situations that may lead to trauma is the best method of prevention. Glasses with strong frames are advised for both protection from ocular trauma and improving vision.
Management
Management of osteogenesis imperfecta requires a multi-disciplinary team due to its systemic manifestation.
Musculoskeletal: Physical examination to assess for fractures, deformities and joint laxity.
- PT, OT, Orthopedics for surgical management
Neurological: If any suspicion for basilar impression due to signs and symptoms, complete CT and/or MRI with views across the base of the skull. Cervical spine flexion and extension radiographs may also assess joint laxity and bone functional status.
Ophthalmic: Ophthalmologist should monitor any changes in the eye, at least yearly, due to increased risk of glaucoma, reduced thickness of corneal and scleral collagen fibers resulting in low ocular rigidity and risk of ocular hypotony.
Dental: Dental examinations are imperative in OI due to the possible mortality from eroding dentition. Children with OI need to have a dental examination by age 2-3 years.
Audiologic: A formal hearing assessment should be conducted on all individuals at diagnosis.
Genetic Counseling: As OI is a genetically inherited disease, genetic study and consultation may assist family planning and/or family management.
Mental Health/Therapy: OI has a severe impact on most patients’ functional abilities. Due to this appropriate psychological therapy and social worker intervention can help improve the quality of life for a patient and their family.
Treatment
Treatment of osteogenesis imperfecta targets reduction of fracture rate, decrease chronic pain, preventing long bone deformities and increasing functional mobility. As the presentation of osteogenesis imperfecta lies on a spectrum, treatment is individualized and requires a multi-modal approach per patient.
Pharmacological treatment
Bisphosphonate therapy is the main pharmacological therapy currently available for most forms of OI; however, outcomes from treatment have varied within the different types of bisphosphonates. Bisphosphonates work by decreasing bone resorption which can increase bone mass, bone strength and result in improved function. It has been used most often in severely affected children. [2]
The Cochrane collaboration’s 2014 update of the OI review indicated that oral and IV bisphosphonate therapy increases bone mineral density in children and adults with this condition, however, there were no differences in their ability to do so between the IV and oral version. This study did not find a consistent decrease in fractures but there was also no evidence of an increase in fracture rate. No evidence was found to increase growth, improve mobility and reduce pain. [1][2][24][25]In a 2019 study by Bains et al., there were findings of increased lumbar vertebral body density and overall reduced rate of fracture and scoliosis in patients treated with bisphosphonates. This study particularly focused on osteogenesis type I. [2][25]
Intravenous pamidronate is the most invasive administration of bisphosphonates but it is used in all forms of OI except for type IV. It has been most studied in infants with severe OI and has been reported to decrease the fracture rate. [1] While less likely, IV pamidronate may have long-term effects on bone union after fracture due to the lower bone turnover. In addition, complications can include transient asymptomatic hypocalcemia and symptomatic hypocalcemia. [1][2]
Surgical treatment
Fractures in OI patients should be treated similarly to children and adults without OI. Given the high rate of fractures periods of immobilization in children with COL1A1/COL1A2 osteogenesis imperfecta should be shortened as much as possible. The casts should be lightweight and small. A collaborative relationship with physical therapy is imperative for the best outcomes with therapy starting as soon as the cast is removed. Intramedullary rodding is the main treatment option to provide anatomic positioning.
If patients are suspected to have a basilar impression, which is a translocation of the upper cervical spine and clivus into the foramen magnum due to skeletal deformity, screening should occur. If the patient has a positive Lhermitte’s sign (sudden electrical shock-like sensation from spine to extremities), neurosurgical management should be initiated to prevent permanent neurological problems.
Patients with OI may develop hearing loss which can be improved with a middle-ear surgery with a prosthetic incus. However, if the hearing loss occurs later in life there may be a sensorineural component that cannot be improved with middle ear surgery. [2]
Prognosis
The prognosis of osteogenesis imperfecta depends on the type of OI and the phenotype from the gene mutation. Osteogenesis Type I is the mildest form with the least impact on a person’s functionality and life span. Osteogenesis Type II is the most lethal, with perinatal fractures and mortality. It is difficult to discern the prognosis for this disease as even within a specific type of OI or specific mutations there is significant variability in the presentation of the disease in each person. [1][2]
Additional Resource
- Steiner RD. COL1A1/2 Osteogenesis Imperfecta. GeneReviews® [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK1295/.Published September 19, 2019. Accessed October 2, 2019.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Sam JE, Dharmalingam M. Osteogenesis Imperfecta. Indian journal of endocrinology and metabolism. https://www.ncbi.nlm.nih.gov/pubmed/29285457. Published 2017. Accessed October 2, 2019.
- ↑ Jump up to: 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 Steiner RD. COL1A1/2 Osteogenesis Imperfecta. GeneReviews® [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK1295/. Published September 19, 2019. Accessed October 2, 2019.
- ↑ Byers, Lee, MI K-K. Osteogenesis Imperfecta. Hereditary Ocular Disease. https://disorders.eyes.arizona.edu/disorders/osteogenesis-imperfecta. Accessed October 2, 2019.
- ↑ Osteogenesis imperfecta - Conditions - GTR - NCBI. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/gtr/conditions/C0029434/. Accessed October 2, 2019.
- ↑ Campagna G, Al-Mohtaseb Z, Khandelwal S, Chang E. Sequential traumatic corneal open globe rupture in a patient with osteogenesis imperfecta type I. American journal of ophthalmology case reports. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6097226/. Published May 6, 2018. Accessed October 2, 2019.
- ↑ Ganesh A, Jenny C, Geyer J, Shouldice M, Levin AV. Retinal hemorrhages in type I osteogenesis imperfecta after minor trauma. Ophthalmology. https://www.ncbi.nlm.nih.gov/pubmed/15234150. Published July 2004. Accessed October 2, 2019.
- ↑ Lagrou LM, Gilbert J, Hannibal M, et al. Altered corneal biomechanical properties in children with osteogenesis imperfecta. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus.
- ↑ Jump up to: 8.0 8.1 Feinstein E, Shapiro J, Francis AW, Chau F. Patient Reported Prevalence of Eye Disease in Osteogenesis Imperfecta. Investigative Ophthalmology & Visual Science. https://iovs.arvojournals.org/article.aspx?articleid=2266825. Published April 30, 2014. Accessed October 2, 2019.
- ↑ Wallace DJ, Chau FY, Santiago-Turla C, et al. Osteogenesis imperfecta and primary open angle glaucoma: genotypic analysis of a new phenotypic association. Molecular vision. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4153423/. Published August 29, 2014. Accessed October 2, 2019.
- ↑ McCain L, Shapiro JR, Podgor J, Kupfer, Rowe. Low ocular rigidity in patients with osteogenesis imperfecta. Investigative Ophthalmology & Visual Science. https://iovs.arvojournals.org/article.aspx?articleid=2176277. Published June 1, 1981. Accessed October 2, 2019.
- ↑ Byers, Lee, MI K-K. Osteogenesis Imperfecta. Hereditary Ocular Disease. https://disorders.eyes.arizona.edu/disorders/osteogenesis-imperfecta. Accessed October 2, 2019.
- ↑ Semra, Guven1 D. Bilateral papilledema in a child with osteogenesis imperfecta. Eye and Vision. https://eandv.biomedcentral.com/articles/10.1186/s40662-016-0056-4. Published October 17, 2016. Accessed October 2, 2019.
- ↑ Chau, F., Wallace, D., Vajaranant, T., Herndon, L., Lee, P., Challa, P., . . . Maumenee, I. (2016, April 06). Osteogenesis Imperfecta and the Eye. Retrieved December 06, 2020, from https://jhu.pure.elsevier.com/en/publications/osteogenesis-imperfecta-and-the-eye-4
- ↑ Jump up to: 14.0 14.1 Osteogenesis imperfecta - Genetics Home Reference - NIH. U.S. National Library of Medicine. https://ghr.nlm.nih.gov/condition/osteogenesis-imperfecta#inheritance. Accessed October 2, 2019.
- ↑ P3H1 gene - Genetics Home Reference - NIH. U.S. National Library of Medicine. https://ghr.nlm.nih.gov/gene/P3H1#synonyms. Accessed October 2, 2019.
- ↑ CRTAP gene - Genetics Home Reference - NIH. U.S. National Library of Medicine. https://ghr.nlm.nih.gov/gene/CRTAP. Accessed October 2, 2019.
- ↑ About OI. (n.d.). Retrieved from https://oif.org/informationcenter/about-oi/.
- ↑ Osteogenesis Imperfecta. (n.d.). Retrieved from https://www.bones.nih.gov/health-info/bone/osteogenesis-imperfecta
- ↑ Steiner RD. COL1A1/2 Osteogenesis Imperfecta. GeneReviews® [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK1295/. Published September 19, 2019. Accessed October 2, 2019.
- ↑ Steiner RD. COL1A1/2 Osteogenesis Imperfecta. GeneReviews® [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK1295/. Published September 19, 2019. Accessed October 2, 2019.
- ↑ Ashournia H, Johansen FT, Folkestad L, Diederichsen ACP, Brixen K. Heart disease in patients with osteogenesis imperfecta - A systematic review. International journal of cardiology. https://www.ncbi.nlm.nih.gov/pubmed/26100571. Published October 1, 2015. Accessed October 2, 2019.
- ↑ Byers, Lee, MI K-K. Osteogenesis Imperfecta. Hereditary Ocular Disease. https://disorders.eyes.arizona.edu/disorders/osteogenesis-imperfecta. Accessed October 2, 2019.
- ↑ McCain L, Shapiro JR, Podgor J, Kupfer, Rowe. Low ocular rigidity in patients with osteogenesis imperfecta. Investigative Ophthalmology & Visual Science. https://iovs.arvojournals.org/article.aspx?articleid=2176277. Published June 1, 1981. Accessed October 2, 2019.
- ↑ Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. The Cochrane database of systematic reviews. https://www.ncbi.nlm.nih.gov/pubmed/25054949. Published July 23, 2014. Accessed October 2, 2019.
- ↑ Jump up to: 25.0 25.1 Bains JS, Carter EM, Citron KP, et al. A Multicenter Observational Cohort Study to Evaluate the Effects of Bisphosphonate Exposure on Bone Mineral Density and Other Health Outcomes in Osteogenesis Imperfecta. JBMR plus. https://www.ncbi.nlm.nih.gov/pubmed/31131341. Published January 7, 2019. Accessed October 2, 2019.