IOL Power Calculation in Ectasia Patients
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Corneal ectasia is a progressive steepening and thinning of the cornea. It is a multifactorial condition. The most common corneal ectasia is keratoconus, but it can also follow a keratorefractive surgery, most commonly excimer laser ablations, radial keratotomies and the most recently released SMILE technique.[1] [2]
Any kind of corneal ectasia will induce, as it progresses, myopia and an irregular astigmatism with a wide variation in power within small areas. The degree of the myopia and corneal astigmatism will be in direct relation with the ectasia progression and change in the corneal shape.
IOL calculation in these eyes is a more challenging process. This is due to the changes in the cornea with a wide range of K values in a small area, and the changes in the anterior chamber which makes it more difficult to calculate the effective lens position. Nowadays there is a wide variety of intra ocular lens options, and we have seen publications reporting good results with toric IOL’s in ectatic eyes. Always remember that a patient with a toric IOL will not be able to use a Toric contact lens to correct the remaining astigmatism.[3]
The two most common corneal ectasias in which we will have to calculate an intra ocular lens are Keratoconus and post refractive surgery ectasia. The last one resembles keratoconus in what this topic concerns, meaning that for IOL calculation we will think in all the different corneal ectasias as only one entity.
Stability
For us to calculate a lens we need to be confident with the corneal stability. It is not a good idea to calculate a lens in a fluctuating cornea. If we do so, the end result will not be the desired result. It is always correct to advise patients about the small risk of corneal decompensation and ectatic progression after surgery. These corneas have a reduction in biomechanical strength resulting in insufficient corneal integrity to resist deformation,[4] so the same problem that caused the condition can cause a new beginning of progression post-surgery. If the corneal ectasia is progressing, we might want to cross link the cornea and stabilize it before calculating a lens. To rule out a progressing ectasia my recommendation is to perform 3 corneal tomographies 3 to 6 months apart from each other, depending on individual cases.
Clinical Exam
When we receive a patient with a corneal ectasia referred for cataract surgery, the first thing we need to do is a good manifest refraction. The manifest refraction will give us and the patient an idea of expectation; what the result can be after surgery and if the cataract surgery is the logical first step or if we should evaluate other options before. As we mentioned earlier, it is of good practice to ensure stability before proceeding. Remember that the VA might be also affected by the cataract, and not only the corneal ectasia. A hard contact over refraction will also help to determine if the decreased visual acuity is due to the cornea or the cataract. If a hard contact lens improves the vision, the decreased vision is due to the ectasia. If the hard contact lens does not improve the vision, then the cataract is to blame. We will not rely on a manifest refraction to decide an axis for a toric IOL, in fact that axis might be influenced by certain amount of internal astigmatism. If the patient gets a good BCVA on a manifest refraction, we can think that the patient will have a good UCVA post-surgery.
We will also check the corneal clarity and deduct what amount of visual affection is due to the cataract or to the corneal ectasia itself. If the cornea is not clear enough for the patient to have a good VA after surgery, we might want to think other options; for example a keratoplasty or a triple procedure.
Biometry
Three basic measurements are included in all the different formulas, the axial length, the corneal power and the estimated lens position. These measurements are more challenging to accurately acquire in ectatic corneas for reasons we are going to explain.
There are many different devices for us to calculate the values we need for our formulas. These different devices include the manual keratometers, automatic keratometers, IOLMaster, Lenstar, etc. The “all in one” devices for IOL calculations (IOLMaster, Lenstar, etc.) are designed to measure with precision normal eyes and the most common variations. Newer devices are improving their accuracy with longer and shorter eyes, but the measurements in corneal ectasias are still to be improved.
The steeper cornea and off-centered apex makes it difficult to obtain accurate keratometry readings.[3][5] Ectatic eyes have higher axial lengths and deeper anterior chambers,[6]making the effective lens position harder to estimate.[5] As the apex is off-center it might not be in the visual axis, this means that the corneal k’s measured in the apex of the cornea might not be the k’s we have to use for our calculations. In addition, we have to decide what optical zone we are going to use. There is no specific rule to decide how large this zone should be; have in mind that larger optical zones will overestimate k’s including the off-centered corneal apex in the calculation.[3]
Ultrasound and Optical Biometry
Ultrasound biometry
Ultrasound biometry has proved to be inaccurate in ectatic patients.[7] Optical biometry is preferred for axial length measurements due to the acquisition of it in the visual axis. Dr. Alio et al enforced the importance of the accuracy of the AL in these patients showing a strong correlation between the pre-operative axial length and the post-operative spherical equivalent.[8]
Optical Biometry
The most known devices are the PCI devices (IOL Master) and OLCR devices (Lenstar).
- IOLMaster (Carl Zeiss Meditec AG, Jena, Germany): it was introduced in 1999 by Carl Zaiss. It is based on Partial coherence interferometry; its latest version, the IOLMaster 700, incorporates the sweep source OCT. [9]It is an all in one device measuring all the necessary data for IOL calculation. With ectatic eyes we will not consider this as an absolute truth; in fact, we might want to compare our K’s with other devices (corneal tomography) and maybe use other K’s for our calculations. IOLMaster doesn’t acquire all the measurements in one sweep; this is a disadvantage in ectatic eyes due to the big variations within small areas. Comparing with older generations, the newer device will measure more points in the cornea to calculate the K’s. Until now, we shouldn’t rely in these K’s because as the automated keratometers and corneal topography does, it is only measuring the anterior corneal curvature and making assumptions on the posterior corneal curvature.
- Lenstar (Haag Streit, Koniz, Switzerland): this device uses a technology called Optical low-coherence reflectometry. Its latest version is the LS900. It has the advantage of taking all measurements in a single alignment and a single sweep, improving the accuracy of the individual measurements and increases repeatability. Another advantage is that it incorporates the Barret toric calculator within its formulas. As in the IOL Master, the optical zone were the k values are calculated is good for a normal eye, but small for a patient with a corneal ectasia. It also makes assumptions about the posterior curvature.
Corneal Power
As we mentioned earlier the corneal power is more difficult to calculate in these eyes. It changes widely within small areas due to its great corneal irregularity. An important aspect to understand is that the total corneal power is given by the anterior and posterior cornea, meaning that measuring only the anterior cornea and assuming a fixed value for the posterior cornea will not be accurate enough.
There is a wide variety of devices to measure corneal power, but not all of them are reliable enough for these eyes. The automated and manual keratometry devices, the topography devices, IOLMaster and Lenstar will only measure the anterior corneal surface and make an assumption about the posterior curvature.[10] It has been published that automated keratometry has bias overcorrecting or undercorrecting the corneal astigmatism in WTR (with the rule) astigmatism and ATR (against the rule) astigmatism respectively.[11]Reaffirming the concept, the anterior and posterior corneal measurement is necessary for us to have accurate data.[11][12] [13]
- Manual Keratometry: measures the central 3mm of the cornea, measuring the radius of curvature of the anterior surface and making assumptions regarding the posterior surface. In addition to the data absence concerning to the posterior cornea, the purkinje image will be distorted in ectatic eyes, making the calculations less reliable.
- Automated Keratometry: most devices measure the central 2.8 to 3.33 mm of the cornea. Due to the great variability in diopters within different areas in these corneas, measuring only one small area is not enough to have accurate results. Automated Keratometry has a better repeatability than the manual keratometry but we basically find the same problems as in manual keratometry.
- Corneal Topography: It measures anterior corneal curvature and it will calculate elevation using Slope. It will make assumptions on the posterior corneal curvature assigning to it a fixed value. Ectatic corneas have multiple different elevations therefore calculating elevation from slope will not be accurate.
- Corneal Tomography: There is no doubt that up to now these devices are the gold standard to calculate K’s in ectatic corneas. The main difference with the other devices is that it measures elevation in a direct manner, and it calculates curvature from it. The most commonly used devices are the Pentacam (Oculus GmbH, Wetzlar, Germany) and Galilei (Ziemer Ophthalmic Systems AG); both use the Scheimpflug technology to obtain their images. The Oculus Pentacam has one rotating camera, directly measuring elevation and calculating curvature. The Galilei has two rotating cameras and a built-in Placido disc, calculating corneal curvature by merging the Placido and the elevation data. With the tomography we are measuring the posterior corneal curvature and calculating the total corneal power without assumptions.
Scheimpflug Devices
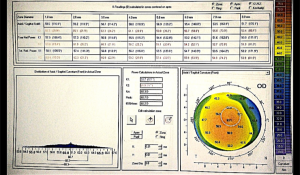
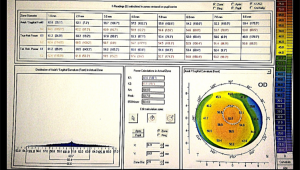
When using these devices, we can overcome the problem of the off-centered corneal apex for K’s calculation. As I mentioned before we have an option to choose between a centration in the “Apex” (figure 1) or the “Pupil” (figure 2). This is useful because the apex might not be within the visual axis, and if we consider the high K’s from it we will underestimate the power of the lens.
As mentioned earlier, these devices have the advantage of measuring elevation of the anterior and posterior corneal curvatures. Two maps generated from these devices of great value for us are: the True net power map and the Equivalent K-Readings (EKR).
True net power map
True net power map will measure the refractive power of the anterior and posterior cornea assigning to each of them specific refractive indices. Then, it will put together many k readings in the area that we select, making it easier to select the k’s we want to use for our IOL calculation.
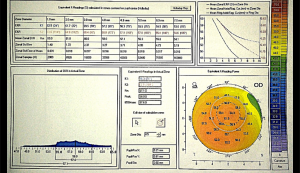
Equivalent K-Readings (EKR)
EKR will output the information in the way keratometers do (SimK), but including in the equation the anterior and posterior corneal power. EKR corrects keratometric values measured by the tomographer and adds the error that would be created using a single refraction index (1.3375) in a sagittal map. It allows us to evaluate the progression of power between the different areas and decide the K’s we want to use in the area we desire between 1 to 7 mm (Figure 3).
Formulas
Most of the formulas we currently have are optimized for eye models that do not resemble an eye with a corneal ectasia, and there is no large study clarifying which formula is the best for these patients.[10]Due to the off-centered axis, optical measurement devices are preferred instead of ultrasound devices.[3]The OLCR device in combination with the Barret toric calculator showed the best corneal astigmatism prediction error compared with a PCI device and other formulas.[14]Dr D. Cooke showed in his publication comparing preinstalled formulas in PCI and OLCR devices that OLCR devices in combination with Olcen formula has the best results for long eyes.[15] Thebpatiphat et al. did a small study comparing three formulas, SRK, SRK-II and SRK-T and he concluded that the SRK-II formula might be the most accurate in mild Keratoconus.[16] Dr. S Bozorg and R Pineda in their review suggested that the Holladay 2 formula might be more accurate than other formulas when effective lens position is variable.[10] Dr. Alio et al did a retrospective study in which he operated with MICS and inserted toric IOL’s in 17 eyes of 10 patients. He reported good results using the Hoffer Q formula in patients with average axial lengths and the SRK/T formula in longer eyes, with the last one having the best results.[8] Newer generation formulas like Barret Universal II, and the RBF calculator are promising but larger studies in ectatic corneas are needed.
IOL Options
The most widely used lenses for ectatic patients are the monofocal lenses. This is because we want to try not to induce more aberrations and, if we can, decrease them. Multifocal lenses are not recommended due to the high amount of higher order aberrations these corneas have; we would induce more aberrations and negatively affect the end result. Toric IOLs are becoming a popular option, but we have to know that for high astigmatisms in ectatic patients we will be debulking the astigmatism but not canceling it 100%. Toric IOLs are an option for carefully selected patients, and we should always have a detailed conversation in order to avoid unreasonable expectations and a good understanding of complications. We are going to offer a toric IOL only to patients that will not use toric contact lenses after the surgery.
Toric IOLs
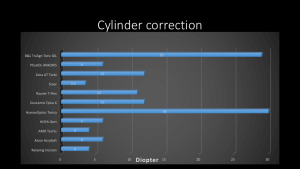
With the new technologies and high cylinder IOL’s the spectrum of patients that we can correct is wider and the results are improving. In some patients with mild ectasia we can obtain perfect refractive results. In other patients with a more advanced ectasia and more irregular astigmatism we might be able to debulk the cylinder for the patient to acquire a workable UCVA. In figure 4 we can see a wide variety of toric IOL’s; we can correct up to 30D of corneal cylinder. Not all of them are FDA approved (Figure 4).
New Technologies
Newer devices are being released to improve corneal power measurement and improve IOL calculations. Some of these new technologies are the Pentacam AXL which adds to the corneal tomography device the axial length measurement and IOL calculation for spherical and toric IOL’s. Another device is the Optovue Cornea Advance; this can measure corneal power getting direct anterior and posterior corneal curvature measurements by OCT technology.
Advancements in devices and IOL technology are making calculations safer and more predictable increasing the expectations of surgeons and patients.
Useful Resources
- http://www.ascrs.org/barrett-toric-calculator
- http://www.apacrs.org/barrett_universal2/
- http://rbfcalculator.com/
References
- ↑ Gitansha Sachdev, MS, FICO, Mahipal S. Sachdev, MD, Ritika Sachdev, MS, Hemlata Gupta, MS, DNB, FAICO. Unilateral corneal ectasia following small-incision lenticule extraction. J Cataract Refract Surg 2015; 41:2014–2018 Q 2015 ASCRS and ESCRS.
- ↑ Mohamed Tarek El-Naggar, MD, FRCS. Bilateral ectasia after femtosecond laser–assisted small-incision lenticule extraction. J Cataract Refract Surg 2015; 41:884–888 Q 2015 ASCRS and ESCRS.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Jean-Louis Bourges (2012). Cataract Surgery in Keratoconus with Irregular Astigmatism, Astigmatism - Optics, Physiology and Management, Dr. Michael Goggin (Ed.), InTech, DOI: 10.5772/20522. Available from: http://www.intechopen.com/books/astigmatism-optics-physiology-and-management/cataract-surgery-on-irregular-astigmatism-of-keratoconus.
- ↑ Dawson DG, O’Brien TP, Dubovy SR, et al. Post-Lasik ectasia: histopathology, ultrastructure, and corneal physiology from human corneal buttons and eye bank donnors. Presented at the AAO Annual Meeting, Las Vegas, NV 2006.
- ↑ Jump up to: 5.0 5.1 Divya Srikumaran, MD. How to approach cataract surgery in keratoconus. Opthalmology Times, May 15, 2013.
- ↑ Ille ́s Kova ́cs, MD, PhD, Kata Miha ́ltz, MD, Ja ́nos Ne ́meth, MD, DSc, Zolta ́n Z. Nagy, MD, DSc. Anterior chamber characteristics of keratoconus assessed by rotating Scheimpflug imaging. Cataract Refract Surg 2010; 36:1101–1106 Q 2010 ASCRS and ESCRS.
- ↑ Antonio Leccisotti, MD, PhD. Refractive lens exchange in keratoconus. J Cataract Refract Surg; 32:742-746, 2016 ASCRS and ESCRS.
- ↑ Jump up to: 8.0 8.1 Alio JL, Garcia PP, Guliyeve FA, et al. MICS with toric intraocular lens in keratoconus: outcomes and predictability analysis of postoperative refraction. Br J Ophthalmol 2014; 98 (3):365-370.
- ↑ 18 Akman A, Asena L, Güngör SG. Evaluation and comparison of the new swept source OCT-based IOLMaster 700 with the IOLMaster 500. Br J Ophthalmol. 2016 Sep;100(9):1201-5. doi:10.1136/ bjophthalmol-2015-307779.
- ↑ Jump up to: 10.0 10.1 10.2 Sara Bozorog, Roberto Pineda. Cataract and keratoconus: Minimizing complications in intraocular lens calculations. Seminars in Ophthalmology. 2014; 29 (5-6): 376-379.
- ↑ Jump up to: 11.0 11.1 Lijun Zhang, MD, Mary Ellen Sy, MD, Harry Mai, BA, Fei Yu, PhD, D. Rex Hamilton, MD, MS. Effect of posterior corneal astigmatism on refractive outcomes after toric intraocular lens implantation. J Cataract Refract Surg 2015; 41:84–89 Q 2015 ASCRS and ESCRS.
- ↑ Hung Lee, Tae-im Kim, and Eung Kweon Kim. Corneal astigmatism analysis for toric intraocular lens implantation: precise measurement for perfect correction. Curr Opin Ophthalmol 2015, 26:34-38.
- ↑ Gabor Nemeth, MD, PhD, Andras Berta, MD, PhD, DSc, Agnes Lipecz, MD, Ziad Hassan, MD, PhD, Eszter Szalai, MD, PhD, and Laszlo Modis Jr, MD, PhD, DSc. Evaluation of Posterior Astigmatism Measured with Scheimpflug Imaging. Cornea 2014;33:1214–1218.
- ↑ Adi Abulafia, MD, Graham D. Barrett, MD, Guy Kleinmann, MD, Shay Ofir, MD, Adi Levy, BSc, Arie L. Marcovich, MD, PhD, Adi Michaeli, MD, Douglas D. Koch, MD, Li Wang, MD, PhD, Ehud I. Assia, MD. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg 2015; May;41(5):936-44.
- ↑ David L. Cooke, MD, Timothy L. Cooke, BA. Prediction accuracy of preinstalled formulas on 2 optical biometers. J Cataract Refract Surg 2016; 42:358–362 Q 2016 ASCRS and ESCRS.
- ↑ Thebpatiphat N, Hammersmith KM, Rapuano CJ, et al. Cataract surgery in keratoconus. Eye Contact Lens 2007; 33:244–246.
- Efstratios A. Parikakis, Irini P. Chatziralli, Vasileios G. Peponis, Georgios David, Spyridon Chalkiadakis, Panagiotis G. Mitropoulos. Toric Intraocular Lens Implantation for Correction of Astigmatism in Cataract Patients with Corneal Ectasia. Case Rep Ophthalmol 2013;4:219–228.
- Jarade E, Dirani A, Fadlallah AMD, Chelala E, Fakhoury H, Cherfan G. Intraocular Lens Power Calculation after Intracorneal Ring Segment Surgery for the Treatment of Post-LASIK Ectasia. SM Opthalmol J. 2015;1(1):1005.