All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
High altitude retinopathy (HAR) encompasses a spectrum of pathological retinal changes occurring in unacclimatized individuals exposed to hypobaric hypoxia encountered at high altitudes. Affected individuals are usually asymptomatic and typical features, including retinal hemorrhages and papilledema, generally resolve spontaneously without adverse visual outcomes on descent to lower altitudes.
Disease Entity
ICD-10:
- H35.09 - other intra-retinal microvascular abnormalities
- T70.29 - other effects of high altitude
Disease
High altitude retinopathy (HAR) is one of four clinical entities of high-altitude illness (HAI) that includes acute mountain sickness (AMS), high altitude cerebral edema (HACE) and high altitude pulmonary edema (HAPE). HAI occurs with travel to elevations above 2500 meters (~8200 feet) and is generally characterized by symptoms of headache, nausea, vomiting and tiredness, but may affect the lungs, brain, and eyes differently among individuals.[1]
HAR encompasses a spectrum of pathological retinal changes occurring in unacclimatized individuals exposed to hypobaric hypoxia encountered at high altitudes and may reflect individual susceptibility to pathology.
Etiology
Various vascular compensatory mechanisms and pathological responses induced by hypobaric hypoxia, and potentially exacerbated by physical exertion and Valsalva effects, are believed to cause physiological and pathological retinal changes observed in individuals who ascend to high altitudes.[2][3]
Risk Factors
Altitude illness
Independent risk factors for the development of altitude illness include:[5][6]
- higher ascent
- rate of ascent
- individual susceptibility
High altitude retinopathy
Specific risk factors for HAR include:[7][4][8]
- higher ascent
- longer duration at altitude
- lower oxygenation saturation by pulse oximetry (SpO2)
- higher hematocrit;
- higher baseline intra-ocular pressure (IOP)
HAR has also been noted to occur more frequently in young and physically well-trained individuals, especially those undergoing strenuous activity at high altitudes. This is thought to be due to the higher likelihood of these individuals undergoing such activity. [9][10]
Typically, HAR occurs in patients who ascend to altitudes above 12,000 feet. [3] However, HAR can occur with ascents to altitudes less than 12,000 feet, especially in individuals with other risk factors, such as dehydration [11] or pre-existing medical conditions. For example, a patient with cystic fibrosis was reported to develop retinal vessel tortuosity and hemorrhage during a hiking trip at 4,900-9,800 feet.[12]
There has also been at least two reported cases of high altitude retinopathy associated with prolonged commercial flights, although this appears to be rare.[13][14]
Use of non-steroidal anti-inflammatory drugs (NSAIDs) has been implicated as a possible risk factor for HAR, but studies are contradictory.[4][8]
Of note, despite an association between low birth weight and mothers living at altitude, there is no significant association between altitude and retinopathy of prematurity in premature infants.[15]
Physiology
Systemic
Hypobaric hypoxia experienced at high altitudes induces several of compensatory physiologic mechanisms to counteract the resultant hypoxemia. Such homeostatic mechanisms include an increase in cardiac output and ventilation; modification of blood flow by local and systemic auto-regulatory adjustments in regional vascular systems; a right shift of the oxygen-hemoglobin dissociation curve; and a later secondary polycythemia resulting in an increased hemoglobin concentration and hematocrit.[10]
Regional
Increased retinal blood flow, decreased retinal circulation time, retinal vasodilation and an absolute increase in retinal vascular blood volume are important auto-regulatory responses aimed at maintaining tissue oxygenation in eyes exposed to chronic hypoxia.[16] These regional compensatory responses typically cause a clinically observable increase in the diameter and tortuosity of retinal vessels and disc hyperemia, which are considered normal responses and are seen in almost all individuals who live at high altitude.[2][7] Choroidal vessels can also auto-regulate but early studies suggest that choroidal vessels do not operate normally at full-flow capacity.[17] Enhanced depth imaging spectral domain optical coherence tomography (EDI SDOCT) has shown a small increase in choroidal thickness with acute high altitude exposure which was fully reversible upon return to low altitude.[18]
Long Term Altitude Exposure
Individuals who live at high altitude long term have shown changes that are not associated with retinopathy, including an alteration in central foveal thickness.[19] Even among individuals who live at high altitude, oxygen concentrations have been noted to improve on descent to sea level.[20]
Pathophysiology
The development of HAR is hypothesized to involve systemic hypoxic effects on the eye despite regulatory attempts by the retinal circulation to achieve homeostasis. In addition to the physiological changes mentioned above, various superimposed pathological factors are thought to contribute to the development of HAR.[21] However, due to difficulties with experimentation at high altitude, the following may be based on retinal pathophysiology at normal oxygen concentrations and should be carefully considered as potentially hypothetical:
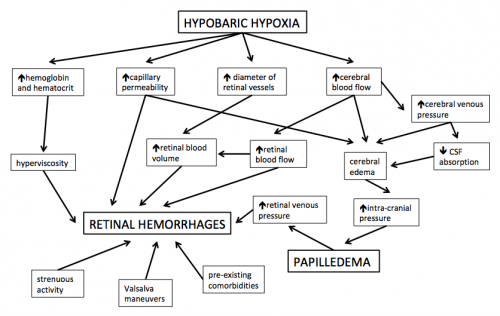
- Increased expression of nitric oxide (NO) and vascular endothelial growth factor (VEGF) cause a breakdown of the inner blood-retinal barrier, leading to vasodilation, increased vascular permeability and capillary proliferation.[22][23]
- Increased hematocrit and hemoglobin concentration leads to increased blood viscosity, increased coagulability and decreased oxygen transport capacity.[9]
- Elevated blood viscosity may increase shear stress to the pre-damaged vascular endothelial cells.[24]
- Hypoxia-induced increase in cerebral blood flow and decreased blood-brain barrier integrity result in cerebral edema, leading to an increase in brain volume and optic disc swelling (papilledema).[25][21]
- Cerebral edema, pulmonary edema and local factors may all lead to an increase in retinal venous pressures.[2][3][16]
- Coughing, vomiting, straining, strenuous exercise and other Valsalva-like maneuvers further increase intra-vascular pressures.[2][3][16]
- Local stasis caused by impaired micro-circulation and local high vascular pressure peaks may lead to capillary bursts.[2][3]
- Pre-existing medical conditions may exacerbate retinal vessel fragility (e.g. retinal microvascular diseases, such as diabetic or hypertensive retinopathy), coagulability (e.g. anti-phospholipid syndrome) or systemic hypoxia (e.g. chronic obstructive pulmonary disease or cystic fibrosis).[7][24][26][27][28][29]
- Genetic susceptibility to the effects of high altitude, or protection against high altitude, may also play a role in the pathophysiology of HAR.[30]
General pathology
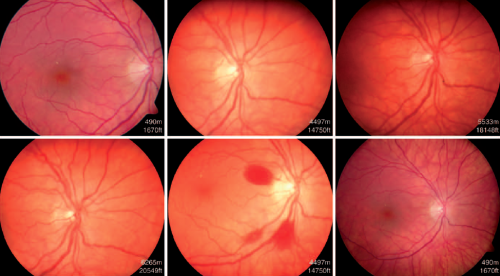
Retinal hemorrhages from capillary bursts or leakage from the arterial side of the retinal vascular bed are a predominant pathological finding in HAR.[7][31] These hemorrhages may be punctate or diffuse, intra-retinal or pre-retinal, and peripheral or central. The cause of the bleeding is not clear but one consideration is that low oxygen from shunting of blood or hypoperfused capillary beds stimulates expression of factors that increase vascular permeability, such as VEGF.
Small hemorrhages are initially punctate and intra-retinal with a flame-shaped or feathery appearance, following the anatomy of the nerve fiber layer.[24] More extensive vessel damage may result in larger or diffuse hemorrhages, which tend to be more prominent and well-defined and are often situated pre-retinal.[24] In cases where the posterior vitreous is not adherent to retina, bleeding may be dispersed into the vitreous cavity. [24] Retinal hemorrhages tend to occur peripherally but occasionally may involve the macula.[16] Differences in regional retinal blood flow and arteriovenous transit time are hypothesized to be the reason for this clinical finding.[16]
Hemorrhage, hypoxia, hypoperfusion, fluctuations in vascular pressures and vascular dysfunction may all contribute to retinal nerve fiber layer infarcts, disruption of axoplasmic flow (resulting in cotton wool spots), micro-embolization of platelet aggregates (resulting in Roth spots) and retinal vein occlusions.[7][4][8][32]
Increased intra-cranial pressure (ICP) as a result of hypoxia-induced changes in cerebral blood flow and blood-brain barrier function lead to papilledema.[24][25]
Vision loss in the setting of HAR may be the consequence of::
- macular or para-macular retinal hemorrhages
- vitreous hemorrhage
- retinal nerve fiber layer infarcts
- macular edema
- macula ischemia
- optic atrophy
Molecular mechanisms
Scientific studies on molecular mechanisms of HAR are sparse.
A 2021 study by Su et al[33] identified genes and microRNAs (miRNAs) that are potential biomarkers for HAR using a series of integrated bioinformatics analyses and experiments involving hypoxia-induced cell culture. Human retinal microvascular endothelial cells (HRMECs) were cultured in a hypoxia chamber filled with an anaerobic gas mixture of 95% N2, 5% CO2, and 1% O2 for 12 hours. Control groups were cultured in 95% air and 5% CO2. The authors selected candidate HAR-related miRNAs and mRNAs by analyzing pre-existing datasets of acute mountain sickness (AMS) obtained from the gene Expression Omnibus (GEO). These included the miRNA expression profile GSE90500 and the mRNA expression profile GSE75665 obtained from blood samples of patients with acute mountain sickness (AMS) and volunteers without AMS. The study identified FOS, IL10, IL7R mRNAs, and seven miRNAs as candidate biomarkers of HAR. The expression of miR-3177-3p, miR-369-3p, miR-603, miR-495-3p, miR-495-5p, miR-4791, and miR-424-5p were significantly up-regulated in HRMECs cultured in hypoxia compared to normoxia. FOS, IL10, and IL7R were strongly down-regulated under hypoxic conditions. Further studies are required to determine if these findings are relevant in HAR.
Another study in 2017 by Xin et al[34] explored the effect of hypobaric hypoxia on the rat retina and determined whether resveratrol had a protective effect on hypoxia-induced retinal damage. The study reported that hypobaric hypoxia increased the expression of thioredoxin 1 (Trx1) and thioredoxin 2 (Trx2) in the retina, and resveratrol treatment significantly inhibited the increased expression levels. In addition, hypoxia led to upregulated mRNA expression of caspase3, caspase9, heat shock protein 70 (Hsp70), heat shock protein 90 (Hsp90) and hypoxia-inducible factor-1 (HIF-1). Resveratrol significantly decreased the expression of caspase3, HSP90, and HIF-1 mRNAs. The investigators proposed that resveratrol acted as an anti-oxidant by modulating hypoxia-induced stress-associated gene expression levels and as an anti-apoptotic agent by regulating apoptosis-related cytokine mRNAs. The authors suggested that resveratrol might have a role in the prevention and treatment of high-altitude retinopathy.
Prevention
Primary
Avoidance of ascent to high altitude.
Secondary
The severity of HAR may be mitigated by acclimatization, slower ascent, maintenance of higher SpO2 (e.g. with supplemental oxygen) and avoidance of strenuous activities, Valsalva maneuvers and NSAIDs while at high altitude.[7][4][8][9][10]If symptoms of high altitude such as visual disturbances, nausea and headaches occur, descent to lower altitude may be considered and is recommended. Treatment for comorbid conditions (i.e. obstructive sleep apnea [35]) that put patients at risk for retinal or optic nerve vascular events has also been suggested, especially with repeated high-altitude exposure for patients particularly at risk.
Special Populations
High-altitude hypobaric hypoxia impacts the function of the macular region, and this suggests that persons with age-related macular degeneration, tapetoretinal degeneration or diabetic retinopathy should pay particular attention to proper acclimatization procedures and avoid extended exposure to high altitude.[18] Patients with Sickle cell trait may also be at a higher susceptibility for proliferative retinopathy associated with high altitude exposure.[36] People with high altitude polycythemia are also at higher risks for retinal and choroidal microcirculation disturbances.[37]
Diagnosis
History
Current or recent ascent to high altitude, with or without associated visual complaints. It has also been reported after a high-altitude flight. [38]
Physical examination
Clinical features of HAR are apparent on dilated fundus examination.
Signs
Description
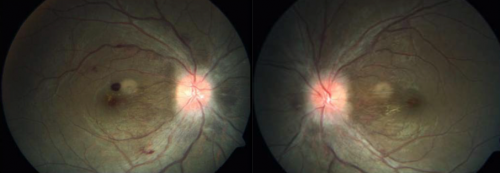
Signs of HAR were first described by Singh in 1969, typical features include:[40]
- increasingly dilated retinal vessels (veins and arteries)
- papillary hemorrhage [42]
- peri-papillary hyperemia [44]
- papilledema
Cotton wool spots, Roth spots and retinal vein occlusions (both branch and central) have also been described in association with HAR.[6][4][8][9] Central retinal artery occlusion has also been reported, [45] as well as being associated with optic neuropathy including non-arteritic anterior ischemic optic neuropathy (NAION). [35]
These findings may persist for weeks after return to baseline altitude and resolve with time. [46][41]
Barthelemes et al[4] published a case series of 28 patients, who underwent fundus photography along their ascent and descent of Mt. Austagh Ata (24, 751 ft). Few retinal hemorrhages were noted during the ascent. However, retinal hemorrhages increased during the descent. In this study, 79% of patients exhibited retinal hemorrhages, most of which were detected after return to base camp from a high altitude. Significant correlations with decreased oxygen saturation and increased hematocrit were noted. Due to the delayed appearance, the authors suggested that retinal hemorrhages should not be considered a warning sign of impending high-altitude illness. This finding is consistent with Singh et al’s observational study, which demonstrated a time lag of six to 96 hours between arrival and onset of symptoms of acute mountain sickness (AMS).[40] The time lag between arrival of high altitude and onset of signs and/or symptoms suggest HAR or AMS may not be directly or solely related to hypoxia.
Classification
Wiedman and Tabin established a classification system for HAR in 1999, based on progressive severity of clinical signs:[47]
- Grade I
- A: Mildly dilated retinal veins (venule:arteriole ratio 3:2)
- B: Retinal hemorrhages up to one disc area
- Grade II
- A: Moderately dilated retinal veins (venule:arteriole ratio 3.5:2)
- B: Retinal hemorrhages up to two disc areas
- Grade III
- A: Greatly dilated retinal veins (venule:arteriole ratio 4:2)
- B: 1) Retinal hemorrhages up to three disc areas; 2) para-macular retinal hemorrhages; 3) vitreous hemorrhage (minor, <3 disc areas)
- Grade IV
- A: Engorged retinal veins (venule:arteriole ratio 4.5:2)
- B: 1) Retinal hemorrhages more than three disc areas; 2) macular retinal hemorrhages; 3) vitreous hemorrhage (major, >3 disc areas); 4) papilledema
Symptoms
Affected individuals are usually asymptomatic. Infrequent symptoms may include: sudden painless onset of floaters, blurring and/or loss of vision.
Clinical diagnosis
Based on the identification of typical retinal changes in an individual with current or recent ascent to high altitude. May be an incidental finding or be prompted by the onset of new visual complaints.
Diagnostic procedures
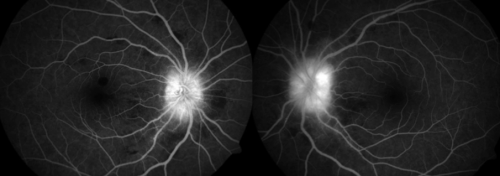
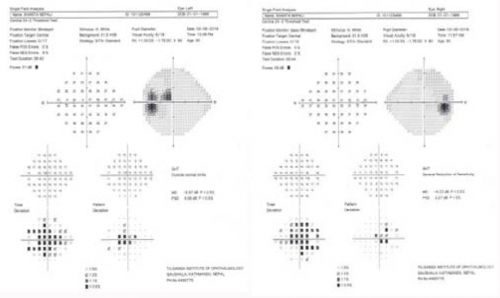
High altitude retinopathy (HAR) is predominately a clinical diagnosis but ophthalmic imaging and visual field testing have been used to define the extent, severity and complications of the condition, as well as to monitor for follow up. These include:
- fundus photography
- fluorescein angiography
- optical coherence tomography
- perimetry and visual field analysis
Laboratory tests
Blood tests are generally not indicated for diagnostic purposes but may reveal elevated hemoglobin concentration and hematocrit.
Differential diagnosis
- Diabetic retinopathy
- Hypertensive retinopathy
- Valsalva retinopathy
- Terson syndrome
- Retinal vein occlusion from other causes
- Cotton wool spots from other causes
- Roth spots from other causes
- Papilledema from other causes
Management
General treatment
High altitude retinopathy (HAR) is usually asymptomatic, and typical retinal changes are often only detected incidentally after descent to lower altitudes or not detected at all. If vision-involving HAR develops at high altitude, general treatment measures include immediate descent to lower altitudes and administration of supplemental oxygen.[16]
Medical therapy
The majority of cases of HAR resolve spontaneously with no long-term adverse visual outcomes.[7][4] Besides the general measures mentioned above, no specific therapies for HAR have been proven, although it may be necessary to treat the acute manifestations of altitude illness or the chronic ocular complications of the condition (e.g. retinal vein occlusion). Medications such as NSAIDs, corticosteroids and acetazolamide have been shown to have no impact on retinal hemorrhages[4] The use of furosemide is inconclusive.[32]
Although no systemic medications are indicated specifically for HAR, many are suggested for the other three clinical entities of high altitude illness (HAI): acute mountain sickness (AMS), high altitude cerebral edema (HACE), and high altitude pulmonary edema (HAPE):[1]
- Carbonic anhydrase inhibitors: acetazolamide and methazolamide
- Steroids: budenoside, prednisone and dexamethasone
- Bronchodilator drugs: salmeterol, theophyline and montelukast
- Selective inhibitor of phosphodiesterase type 5 (PDE5): taladafil and sildenafil
- Calcium modulators: nifedipine and flunarizine
- Non-steroidal anti-inflammatory drugs (NSAIDs) and other analgesics: aspirin, carbasalate and ibuprofen
Medical follow up
Follow up is generally not necessary but may be required in certain cases to monitor and/or treat complications.
Surgery
Surgical management of HAR has not been described.
Complications
Ocular
Documented complications of HAR include:[3][7][4][9][16]
- residual scotomata
- visual field shrinkage
- vision loss from a variety of causes (see General pathology above)
Systemic
Although HAR itself has no systemic complications, the severity of retinopathy has been shown to correlate with the development of high altitude cerebral edema (HACE) and high altitude pulmonary edema (HAPE), and these life-threatening conditions should always be considered in individuals at high altitudes.[9][47] Despite these observations, the usefulness of HAR as a predictive tool for the more severe manifestations of altitude illness remains uncertain.[24]
Prognosis
Figures for both the incidence and visual prognosis of HAR vary widely. HAR has been documented to occur in anywhere between 0-79% of individuals ascending to high altitudes[4][16] Various individual- and ascent-related factors are likely responsible for these disparate findings. Visual prognosis is therefore likely dependent on similar factors. In a single study, 75% of individuals with HAR had full visual recovery at 12 weeks; 19% had only partial visual recovery even at 24 weeks; and 6% showed no visual recovery at 24 weeks.[9] Numerous other studies have reported lower rates of adverse visual outcomes.[2][7][4][8]
Additional Resources
References
- ↑ 1.0 1.1 Nieto Estrada VH, Molano Franco D, Medina RD, Gonzalez Garay AG, Martí-Carvajal AJ, Arevalo-Rodriguez I. Interventions for preventing high altitude illness: Part 1. Commonly-used classes of drugs. Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD009761. doi: 10.1002/14651858.CD009761.pub2. PMID: 28653390; PMCID: PMC6481751.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Rennie D, Morrissey J. Retinal Changes in Himalayan Climbers. Arch Ophthalmol. 1975;93:395-400. doi:10.1001/archopht.1975.01010020409001
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Shults WT, Swan KC. High Altitude Retinopathy in Mountain Climbers. Arch Ophthalmol. 1975;93:404-408. doi:10.1001/archopht.1975.010100204180
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Barthelmes D, Bosch MM, Merz TM, Petrig BL, Truffer F, Bloch KE, et al. Delayed Appearance of High Altitude Retinal Hemorrhages. PLoS ONE. 2011;6(2):e11532. doi:10.1371/journal.pone.0011532
- ↑ Schneider M, Bernasch D, Weymann J, Holle R, Bartsch P. Acute mountain sickness: influence of susceptibility, preexposure, and ascent rate. Med Sci Sports Exerc. 2002;34(12):1886–1891. doi:10.1097/00005768-200212000-00005
- ↑ 6.0 6.1 Bloch KE, Turk AJ, Maggiorini M, Hess T, Merz T, Barthelmes D, et al. Effect of ascent protocol on acute mountain sickness and success at Muztagh Ata, 7546 m. High Alt Med Biol. 2009;10(1):25–32. doi:10.1089/ham.2008.1043
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 McFadden DM, Houston CS, Sutton JR, Powles ACP, Gray GW, Roberts RS. High-Altitude Retinopathy. JAMA. 1981;245:581-586. doi:10.1001/jama.1981.03310310023016
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Butler FK, Harris DJ, Reynolds RD. Altitude Retinopathy on Mount Everest, 1989. Ophthalmology. 1992;99(5):739-746. doi:10.1016/s0161-6420(92)31891-3
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Arora R, Jha KN, Sathian B. Retinal changes in various altitude illnesses. Singapore Med J. 2011;52(9):685-688.
- ↑ 10.0 10.1 10.2 Brinchmann-Hansen O, Myhre K, Sandvik L. Retinal vessel responses to exercise and hypoxia before and after high altitude acclimatisation. Eye. 1989;3:768–776. doi:10.1038/eye.1989.120
- ↑ https://www.illinoisretina.com/blog/case-of-the-month/high-altitude-retinopathy-february-2020/
- ↑ Rimsza ME, Hernried LS, Kaplan AM. Hemorrhagic retinopathy in a patient with cystic fibrosis. Pediatrics. 1978;62:336-338.
- ↑ Okudo AC, Babalola OE. A case of high-altitude retinopathy following long distance air travel to Abuja, Nigeria: Case report. East African Medical Journal. 2022;99(4):4771-4775.
- ↑ Wang Y, Yu X, Liu Z, et al. Influence of hypobaric hypoxic conditions on ocular structure and biological function at high attitudes: a narrative review. Frontiers in Neuroscience. 2023;17. Accessed August 3, 2023. https://www.frontiersin.org/articles/10.3389/fnins.2023.1149664
- ↑ Mehner LC, Wagner BD, Lynch AM. Effects of high altitude on development of retinopathy of prematurity. Investigative Ophthalmology & Visual Science. 2022;63(7):4191-F0251.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 Wiedman M. High altitude retinal hemorrhage. Arch Ophthalmol. 1975;93(6):401–403. doi:10.1001/archopht.1975.01010020415002
- ↑ Bill A, Sperber GO. Control of retinal and choroidal blood flow. Eye. 1990;4(2):319–325. doi:10.1038/eye.1990.43
- ↑ 18.0 18.1 Kalpana BN. Commentary: Altitude, acclimation, arterial alterations in retina and choroid. Indian Journal of Ophthalmology. 2022;70(5):1655. doi:10.4103/ijo.IJO_55_22
- ↑ Gok M, Karaman S, Erdem B. Evaluation of macular and choroidal thickness in healthy residents living at high altitude. Indian J Ophthalmol. 2022;70(5):1650-1655. doi:10.4103/ijo.IJO_2079_21
- ↑ Kofoed PK, Sander B, Zubieta-Calleja G, Kessel L, Klemp K, Larsen M. The Effect of High- to Low-Altitude Adaptation on the Multifocal Electroretinogram. Investigative Ophthalmology & Visual Science. 2009;50(8):3964-3969. doi:10.1167/iovs.08-3216
- ↑ 21.0 21.1 Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8(2):175–191. doi:10.1016/S1474-4422(09)70014-6
- ↑ Kaur C, Sivakumar V, Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci. 2006;47(3):1126–1141. doi:10.1167/iovs.05-0518
- ↑ Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27(6):622–647. doi:10.1016/j.preteyeres.2008.09.003
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 Bosch MM, Barthelmes D, Landau K. High altitude retinal hemorrhages - an update. High Alt Med Biol. 2012;13(4):240–244. doi:10.1089/ham.2012.1077
- ↑ 25.0 25.1 Bosch MM, Barthelmes D, Merz TM, et al. High Incidence of Optic Disc Swelling at Very High Altitudes. Arch Ophthalmol. 2008;126(5):644–650. doi:10.1001/archopht.126.5.644
- ↑ Ho TY, Kao WF, Lee SM, Lin PK, Chen JJ, Liu JH. High-altitude retinopathy after climbing Mount Aconcagua in a group of experienced climbers. Retina. 2011;31(8):1650–1655. doi:10.1097/IAE.0b013e318207ceab
- ↑ Rimsza ME, Hernried LS, Kaplan AM. Hemorrhagic retinopathy in a patient with cystic fibrosis. Pediatrics. 1978;62(3):336–338.
- ↑ Karaküçük S, Mirza GE. Ophthalmological effects of high altitude. Ophthalmic Res. 2000;32(1):30–40. doi:10.1159/000055584
- ↑ Hackett PH, Rennie D. Rales, peripheral edema, retinal hemorrhage and acute mountain sickness. Am J Med. 1979;67(2):214–218. doi:10.1016/0002-9343(79)90393-0
- ↑ Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, et al. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 2014;46(9):951-956. doi: 10.1038/ng.3067.
- ↑ Frayser R, Gray GW, Houston CS. Control of the retinal circulation at altitude. J Appl Physiol. 1974;37(3):302–304. doi:10.1152/jappl.1974.37.3.302
- ↑ 32.0 32.1 Frayser R, Houston CS, Bryan AC, Rennie ID, Gray G. Retinal hemorrhage at high altitude. N Engl J Med. 1970;282(21):1183–1184. doi:10.1056/NEJM197005212822106
- ↑ Su T, Gu C, Draga D, et al. Integrative analysis of miRNA-mRNA network in high altitude retinopathy by bioinformatics analysis. Biosci Rep. 2021;41(1):BSR20200776. doi:10.1042/BSR20200776.
- ↑ Xin X, Dang H, Zhao X, Wang H. Effects of Hypobaric Hypoxia on Rat Retina and Protective Response of Resveratrol to the Stress. Int J Med Sci. 2017 Aug 17;14(10):943-950. doi: 10.7150/ijms.19391. PMID: 28924365; PMCID: PMC5599917.
- ↑ 35.0 35.1 Liu, Yin A. MD, PhD; Mesentier-Louro, Louise A. PhD; Shariati, Mohammad A. BA; Moss, Heather E. MD, PhD; Beres, Shannon J. MD; Liao, Yaping Joyce MD, PhD. High Altitude as a Risk Factor for the Development of Nonarteritic Anterior Ischemic Optic Neuropathy. Journal of Neuro-Ophthalmology: June 24, 2022 - Volume - Issue - 10.1097/WNO.0000000000001629 doi: 10.1097/WNO.0000000000001629
- ↑ Broadhead GK, Wiley HE, Peprah D, Olumba K, Thavikulwat AT. Proliferative Retinopathy Associated with Repeated High-Altitude Exposure in a Patient with Sickle Cell Trait. High Altitude Medicine & Biology. 2022;23(4):369-371. doi:10.1089/ham.2022.0070
- ↑ Ma J, Niu H, Ma X, Han C, Qu Y. Effects of long-term high-altitude exposure on retinal and choroidal microcirculation. Graefes Arch Clin Exp Ophthalmol. 2022;260(11):3525-3532. doi:10.1007/s00417-022-05699-2
- ↑ Okudo AC, Babalola OE. A case of high-altitude retinopathy following long distance air travel to Abuja, Nigeria: Case report. East African Medical Journal. 2022 Jul 17;99(4):4771-5.
- ↑ 39.0 39.1 39.2 Benjankar M, Ranjitkar EP. Retinal Changes in a Case of High Altitude Retinopathy (HAR). J Clin Ophthalmol Eye Disord. 2019;3(1):1029.
- ↑ 40.0 40.1 Singh I, Khanna PK, Srivastava MC, Lal M, Roy SB, Subramanyam CS. Acute mountain sickness. N Engl J Med. 1969;280(4):175-184. doi:10.1056/NEJM196901232800402
- ↑ 41.0 41.1 Wilczyński M, Kucharczyk M, Filatow S. High-altitude retinopathy–case report. Klinika Oczna/Acta Ophthalmologica Polonica. 2021;116(3):180-3.
- ↑ 42.0 42.1 Z F Liu, X M Pan, G M Wang, H S Bi, High-altitude retinal hemorrhages and macular hemorrhage, QJM: An International Journal of Medicine, Volume 115, Issue 1, January 2022, Pages 45–46, https://doi.org/10.1093/qjmed/hcab282
- ↑ Shrestha A, Suwal R, Shrestha B. Vitreous Hemorrhage following High-Altitude Retinopathy. Case Reports in Ophthalmological Medicine. 2021 Aug 10;2021.
- ↑ Yin X, Li Y, Ma Y, Xie Y, Wang K, Sun D, Liu X, Hao M, Liang M, Zhang S, Guo Y, Jin L, Wang N, Wang J. Thickened Retinal Nerve Fiber Layers Associated With High-Altitude Headache. Front Physiol. 2022 May 4;13:864222. doi: 10.3389/fphys.2022.864222. PMID: 35600299; PMCID: PMC9114875.
- ↑ Nadda D, Sheoran J, Sachar S, Narula A. Central Retinal Artery Occlusion Secondary To High Altitude Exposure. The Official Scientific Journal of Delhi Ophthalmological Society. 2022 Jul 11;32(4):61-5.
- ↑ Maclaren RE, Ikram K, Talks SJ. Fluorescein angiography in altitude retinopathy. Br J Ophthalmol. 2000;84(3):339-400. doi:10.1136/bjo.84.3.337c
- ↑ 47.0 47.1 Wiedman M, Tabin GC. High-altitude Retinopathy and Altitude Illness. Ophthalmology. 1999;106(10):1924-1927. doi:10.1016/S0161-6420(99)90402-5


