Fungal Keratitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Fungal keratitis or keratomycosis refers to an infective process of the cornea caused by any of the multiple pathologic fungi capable of invading the ocular surface. It is most typically a slow, relentless disease that must be differentiated from other types of corneal conditions with similar presentation; especially its bacterial counterpart, which accounts for the majority of the microbial corneal infections.
Disease Entity
Fungal Keratitis
Disease
Fungal keratitis is a serious ocular infection with potentially catastrophic visual results. Caused by any of the many species of fungi capable of colonizing human tissue, its occurs worldwide and its incidence is increasing in frequency.
Etiology
The list covers many fungi including but not limited to yeasts of Candida spp., filamentous with septae such as Aspergillus spp., Fusarium spp., Cladosporium,spp., Curvularia, and non septated such as Rhizopus. Bare in mind that any agent capable of infecting humans is a potential infectious agent, especially if the host has a debilitating disease.
Risk Factors
Risk factors include trauma, ocular surface disease, and topical steroid use. Risks and type of fungi also might vary by geographic location and climate.[1] [2] [3] [4] [5] [6] [7] In warmer climates the rule is that the most common organisms are filamentous fungi, like Fusarium spp and Aspergillus spp. with a strong relationship to trauma. Reports from Brazil show the most common isolates in descending order were Fusarium spp in 67%, Aspergillus spp in 10.5%, and Candida spp in 10%. About 40% of the infections were related to trauma.[1]
In the northern USA, corneal infection by fungus was, until recently, more common in debilitated or immunocompromised patients and the causative organism being a yeast, such as Candida albicans. Filamentous fungi in these latitudes were then rarely reported. A few years ago a breakout of Fusarium keratitis associated with a type of contact lens solution[8] displaced yeasts as the most common fungal corneal infection in some areas. This trend persists in the most recent epidemiological reports. Still they are, in most cases, related to contact lens use.[9] It should be noted that the incidence of contact lens-related fungal keratitis was increasing before the Fusarium outbreak.
This new distribution means that we no longer can rely on the geographical distribution only to initiate empirical treatment. Broad-spectrum treatment should be administered once there is a strong probability of a mycotic infection.
General Pathology
Even though fungi can be classified as a kingdom due to their complexity and unique characteristics, a simple practical classification for ocular infection is used. Under this method, morphology and type of reproductive method define the type of fungi. They are classified for our purpose as yeast, filamentous septated (pigmented and non-pigmented), and filamentous without septae. Fungi are present worldwide and can be part of the ocular flora. They are eukaryotic with a defined nucleus surrounded by a membrane. They can be either saprophytic, free organisms that subsist on decaying organic matter or pathologic and require a living host for perpetuation.
The American Academy of Ophthalmology's Pathology Atlas contains virtual microscopy images of tissue samples with the following:
Pathophysiology
The infection probably starts when the epithelial integrity is broken either due to trauma or ocular surface disease and the organism gains access into the tissue and proliferates. Proteolytic enzymes, fungal antigens and toxins are liberated into the cornea with the resulting necrosis and damage to its architecture thus compromising the eye integrity and function.
Primary Prevention
Wearing safety glasses while gardening will diminish the risk of ocular trauma. Also, general hygiene, proper contact lens care, and avoidance of nonessential steroid use should diminish the probability of mycotic infection.
Diagnosis
A high degree of suspicion from the physician accounts for early diagnosis and treatment, which are paramount for a successful resolution of the fungal keratitis. Corneal ulcers unresponsive to broad-spectrum antibiotics, the presence of satellite lesions, and scanty secretions in a large ulcer are some signs that should raise flags to the attending professional about the possibility of a mycotic agent.
History
Blurred vision, redness, tearing, photophobia, pain, foreign body sensations, secretions related to trauma, ocular surface disease and topical steroid use are all important characteristics to ascertain in the history.
Physical Examination
After establishing the patient's general condition, the examiner should look for evidence of ocular surface disease and determine the amount and type of secretions and lid swelling. The upper eyelid should be everted to exclude a retained foreign body. The examiner should measure the size and depth of the lesion as well as the presence of satellite lesions. The intraocular pressure should also be ascertained. Anterior chamber reaction and evidence of hypopyon should also be recorded. Vitreous reaction if present may suggest intraocular spread of the disease.
Signs
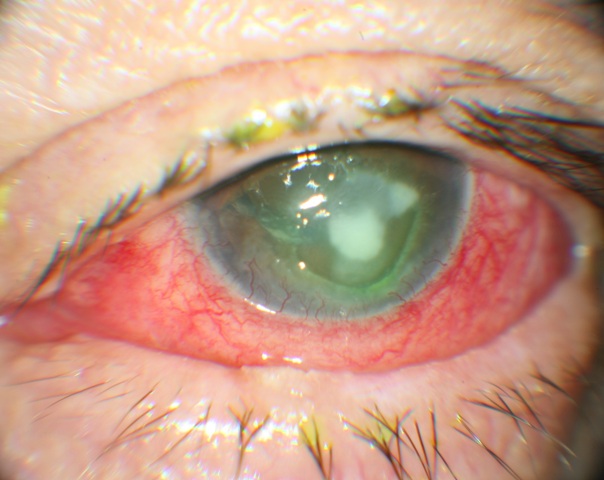
With filamentary fungi, the corneal lesions have a white/gray infiltrate with feathery borders. There might be satellite lesions with a hypopyon and conjunctival injection as well as purulent secretions. Ulcers caused by yeast are plaque-like and slightly more defined, similar to bacterial keratitis.
Symptoms
Symptoms are similar to any corneal infection including blurred vision, redness, tearing, photophobia, pain, foreign body sensation and secretions. In some cases the lesion are rather indolent which help to delay the diagnosis and hence delay the treatment. Suspicion should be high in cases of trauma with vegetable matter.
Clinical Diagnosis
Under the slit lamp, early on the lesion might look like an unhealed corneal abrasion with scanty infiltrates and no secretions. With time however, the ulcer develops thicker infiltrates and fuzzy margins. The presence of satellite lesions strongly suggests a fungal infection. Redness and periocular edema are also common. This combined with a history of trauma, especially with vegetable matter, ocular surface disease (especially neurotrophic keratitis) or chronic use of topical steroids should alert the practitioner to the possibility of a mycotic etiology.
Diagnostic procedures
Corneal scrapings are taken from deep within the lesion with a surgical blade or sterile spatula. To perform a corneal biopsy, a dermatological 2mm punch can be used.
Laboratory test
For a definitive diagnosis, scrapings taken from deep within the lesion should be made and inoculated in Sabouraud agar. The shortcoming is that it can take up to 3 weeks to grow and identify the organism. For a faster result, smears with special stains such as Gomori, PAS, acridine orange, calcofluor white or KOH should be performed. More recently molecular diagnostics have been employed, particularly PCR, has been employed for rapid identification in infectious keratitis cases. The drawback is that not all laboratories have these stains available and not all providers have access to molecular diagnostics, so, again we might need to rely on the patient’s evolution and the physician’s clinical acumen. If all labs and cultures are negative, corneal biopsy should be considered to obtain a specimen.
Differential diagnosis
Fungal infections can mimic any microbial keratitis secondary to other causes: Bacteria, which is the most common cause of corneal infections; Acanthamoeba, related to swimming with contact lenses and or the use of tap water in their cleaning; or herpes simplex or herpes zoster for which recurrences are frequent. Other conditions such as a retained foreign body, sterile infiltrates, marginal ulcers due to Staphylococcal hypersensitivity or chronic epithelial defect should also be ruled out. Again, a high index of suspicion is important in the diagnosis of fungal keratitis. In recent times, there are a large number of cases being reported secondary to Pythium keratitis, which is a close mimicker of fungal keratitis.[10] The features mimicking fungal keratitis are stromal infiltrate, ring ulceration, hypopyon, endothelial exudates and corneal perforation.[11] The classical hallmark feature of Pythium keratitis include reticular dot like infiltrate, tentacular projections, early limbal and peripheral furrowing.[12]
Management
In general, management consists of medical therapy with the use of topical and/or systemic anti-fungal medications alone or in combination with surgical treatment.
General treatment
Topical antifungals, either commercially available or compounded from systemic preparation into eye-drops are the backbone for the management of fungal keratitis. In resistant cases, the addition of systemic antifungal have shown effectiveness. If those treatments fail, then conjunctival flaps , lamellar or penetrating keratoplasty might be needed.
Medical therapy
Fungal ulcers are inherently difficult to treat. The diagnosis is often delayed and medications available for ocular therapy are limited and are deficient in their ability to penetrate deep into the cornea. The mainstay of treatment is the use of antifungal drops but only a polyene, natamycin 5%, is FDA approved and commercially available for topical ocular use. Also, due to the rarity of the diagnosis it is sometimes available only as a special order. Furthermore, the complexity of the disease and possible visually devastating outcomes require a rather aggressive approach to its treatment. Hence, many other antimycotics are used to fight this infection. These compounded eye drops are made by diluting the intravenous medication into concentrations that provide enough medication to eradicate the organism while being tolerated by the eye.
Prior to the development of natamycin the most commonly used antifungal was amphotericin b, a polyene, in a 0.15% dilution in sterile water (one 50mg vial of amphotericin b diluted in 30cc sterile water gives a 0.166% dilution). It is still used today alone and in combination with natamycin with relatively good results. Since they both are readily available, they are both a good choice as initial therapy.
Voriconazole, a triazole antifungal agent derived from fluconazole, can be used either topically at 1% dilution, orally at 400 mg twice a day and even has being injected in the corneal stroma around the fungal lesion (50 micrograms/0.1 ml)[13] [14] [15]
Oral posaconazole, a new generation triazole, has been successful eradicating deep infections of resistant Fusarium[16]Subconjuctival antifungals are not generally used because the produce severe pain and some might even induce tissue necrosis.
Other antifungals available include the azoles like miconazole, clotrimazole ketoconazole, posaconazole, and fluconazole and the echinocandin antifungal agents caspofungin, and micafungin. The echinocandins are not active against Fusarium but have excellent activity against Candida spp including non-albicans Candida.
Care should be taken to provide prophylaxis to bacterial superinfection in the presence of an epithelial defect with broad spectrum antibiotic drops.
Medical follow up
All corneal infections should be followed closely until there is a marked improvement. Since fungal infections run a protracted course, their follow up is longer and after a few days the interval between evaluations increases according to its progress. Complete healing might take weeks and even months. The intraocular pressure should be closed monitored during the episode. It should be noted that epithelialization does not necessary means that the ulcer is healing. In fact it usually hinders the penetration of the fungicide. Confocal microscopy might be an effective additional method to follow the success or failure of the therapy. It does so by direct examination of the organism, inflammation, and corneal stromal cells.[17]
Surgery
Periodic debridement is commonly used in the management fungal keratitis. The procedure removes necrotic tissue and diminishes the organism load but mostly it enhances the penetration of the drugs. It can be performed every 24 to 48 hours.
Role of Intrastromal Injections in Fungal Keratitis discusses this adjuvant therapy in nonhealing keratitis. PACK Crosslinking is also being evaluated as adjuvant therapy to fungal keratitis with mixed results.
If everything fails, a conjunctival flap might deter the infection by providing a rich vascular supply to heal the infection. If there is no response, then a lamellar or penetrating keratoplasty could be needed. If there is a perforation, a patch graft or a therapeutic transplant should be performed. If there is extension of the infiltrate toward the limbus, a therapeutic penetrating keratoplasty should be considered to debulk the infection[18] as well as reduce the technical challenges of a large-diameter graft. The infected cornea should be sent for cultures and pathological evaluation. It is performed in the usual manner but it should extend about 1 to 1.5mm beyond the margins of the lesion.
Surgical follow up
Close follow up for at least 2 weeks with topical antimycotics is recommended. Systemic medication may be added as well. If the edges of the specimen are found by pathology to have organisms the use of topical and systemic antifungals should be extended. Care should be taken to start topical steroids after a therapeutic transplant since it can cause extension of any residual disease. Rather use of cyclosporine topically has been advocated to reduce graft rejection[19].
Complications
Adverse results range from mild to severe corneal scarring, corneal perforation, anterior segment disruption and glaucoma to endophthalmitis resulting in evisceration.
Prognosis
The aftermath of fungal keratitis can be dismal . There is severe visual loss in 26% to 63% of patients. Fifteen to twenty percent may need evisceration. Penetrating keratoplasty is required in 31 to 38% of cases.[1][20]
Additional Resources
- AAO, Basic and Clinical Science Course. Section 8: External Disease and Cornea, 2015-2016.
- AAO, Focal Points: Diagnosis and Management of Fungal Keratitis, Module #6, 2002.
- Keratitis. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/keratitis-list. Accessed March 14, 2019.
- Bron AJ, Seal DV, Hay J. Ocular Infection: Investigation and Treatment in Practice. St Louis: Mosby; 1998.
- O'Brien TP. Therapy of ocular fungal infections. Ophthalmol Clin North Am. 1999;12:33-50.
- O'Day DM. Fungal keratitis. In: Leibowitz HM, Waring GO III, eds. Corneal Disorders: Clinical Diagnosis and Management. 2nd ed. Philadelphia: Saunders; 1998:711-718.
- Prajna NV, John RK, Nirmalan PK, et al. A randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitis. Br J Ophthalmol. 2003;87:1235-7.
- Prajna NV, Krishnan T, Mascarenhas J, et al; Mycotic Ulcer Treatment Trial Group. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013 Apr;131(4):422-9.
- Turbert D, Lipsky SN. Fungal Keratitis. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/fungal-keratitis-list. Accessed March 13, 2019.
References
- ↑ Jump up to: 1.0 1.1 1.2 Ibrahim MM; Vanini R; Ibrahim FM; Fioriti LS; Furlan EM; Provinzano LM; De Castro RS; de Faria E Sousa SJ; Melani Rocha E Epidemiologic aspects and clinical outcome of fungal keratitis in southeastern Brazil. Eur J Ophthalmol. 2009; 19(3):355-61 (ISSN: 1120-6721)
- ↑ Gopinathan U; Garg P; Fernandes M; Sharma S; Athmanathan S; Rao GN The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea. 2002; 21(6):555-9 (ISSN: 0277-3740)
- ↑ Thew MR; Todd B Fungal keratitis in far north Queensland, Australia. Clin Experiment Ophthalmol. 2008; 36(8):721-4 (ISSN: 1442-9071)
- ↑ Ritterband DC; Seedor JA; Shah MK; Koplin RS; McCormick SA Fungal keratitis at the New York Eye and Ear Infirmary. Cornea. 2006; 25(3):264-7 (ISSN: 0277-3740)
- ↑ Jurkunas U; Behlau I; Colby K Fungal keratitis: changing pathogens and risk factors. Cornea. 2009; 28(6):638-43 (ISSN: 1536-4798)
- ↑ Iyer SA; Tuli SS; Wagoner RC Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens. 2006; 32(6):267-71 (ISSN: 1542-2321)
- ↑ Galarreta DJ; Tuft SJ; Ramsay A; Dart JK Fungal keratitis in London: microbiological and clinical evaluation. Cornea. 2007; 26(9):1082-6 (ISSN: 0277-3740)
- ↑ Ma SK; So K; Chung PH; Tsang HF; Chuang SK A multi-country outbreak of fungal keratitis associated with a brand of contact lens solution: the Hong Kong experience. Int J Infect Dis. 2009; 13(4):443-8 (ISSN: 1878-3511)
- ↑ Chang DC, Grant GB, O'Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 2006;296:953-963. D) Prakash G;
- ↑ Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J Ophthalmol. 2021 May;69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. PMID: 33913840; PMCID: PMC8186601
- ↑ Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. Eur J Ophthalmol. 2021 Mar 28:11206721211006564. doi: 10.1177/11206721211006564. Epub ahead of print. PMID: 33779337
- ↑ Gurnani B, Kaur K. Pythium Keratitis. 2021 Jul 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 34424645.
- ↑ Sharma N; Goel M; Titiyal JS; Vajpayee RB Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008; 146(1):56-59 (ISSN: 0002-9394)
- ↑ Thiel MA; Zinkernagel AS; Burhenne J; Kaufmann C; Haefeli WE Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob Agents Chemother. 2007; 51(1):239-44 (ISSN: 0066-4804)
- ↑ Bunya VY; Hammersmith KM; Rapuano CJ; Ayres BD; Cohen EJ Topical and oral voriconazole in the treatment of fungal keratitis. Am J Ophthalmol. 2007; 143(1):151-3 (ISSN: 0002-9394)
- ↑ Tu EY, McCartney DL, Beatty RF, Springer KL, Levy J, Edward D Successful Treatment of Resistant Ocular Fusariosis With Posaconazole (SCH-56592) Am J Ophthalmol. 2007;143:222-227
- ↑ Shi W; Li S; Liu M; Jin H; Xie L Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2008; 246(4):581-6 (ISSN: 0721-832X)
- ↑ Eleiwa, T.K., Youssef, G.H., Elsaadani, I.A. et al. Debulking corneal biopsy with tectonic amniotic membrane transplantation in refractory clinically presumed fungal keratitis. Sci Rep 14, 521 (2024). https://doi.org/10.1038/s41598-023-50987-4
- ↑ Chatterjee S, Agrawal D. Use of Topical Cyclosporine 0.1% in Therapeutic Penetrating Keratoplasty for Fungal Keratitis. Cornea. 2022 Sep 1;41(9):1116-1121. doi: 10.1097/ICO.0000000000002827. Epub 2021 Sep 3. PMID: 34483271.
- ↑ Rondeau N; Bourcier T; Chaumeil C; Borderie V; Touzeau O; Scat Y; Thomas F; Baudouin C; Nordmann JP; Laroche L [Fungal keratitis at the Centre Hospitalier National d'Ophtalmologie des Quinze-Vingts: retrospective study of 19 cases] J Fr Ophtalmol. 2002; 25(9):890-6 (ISSN: 0181-5512)
- Ocular Pathology Atlas. American Academy of Ophthalmology Web site. https://www.aao.org/resident-course/pathology-atlas. Published 2016. Accessed January 4, 2017.