Dysphotopsia
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Unwanted optical images are a leading cause of patient dissatisfaction after uncomplicated cataract surgery. This includes dysphotopsias, or undesirable optical patterns on the retina. Positive dysphotopsia (PD) is a bright artifact of light, described as arcs, streaks, starbursts, rings, or halos occurring centrally or mid-peripherally. Negative dysphotopsia (ND) is the absence of light on a portion of the retina described as a dark, temporal arcing shadow. The exact nature of these events is incompletely understood, but there are many different theories with both clinical and laboratory evidence to support them. At present, it seems that PD is related to intraocular lens (IOL) material, design, and location, while ND is multifactorial in origin, primarily contributing to an illumination gap causing a shadow on the retina. ND is more complex to analyze but may benefit from improved IOL design as well as varying the surgical approach of IOL implantation. While most patient experiences of dysphotopsia resolve or undergo neuroadaptation, persistent symptoms can occur and warrant surgical consideration.
Disease Entity
Disease
Dysphotopsia also known as visual dysphotopsia or pseudophakic dysphotopsia.
- Other visual disturbances ICD-10 H53.8
- Other subjective visual disturbances ICD-10 H53.19
Etiology
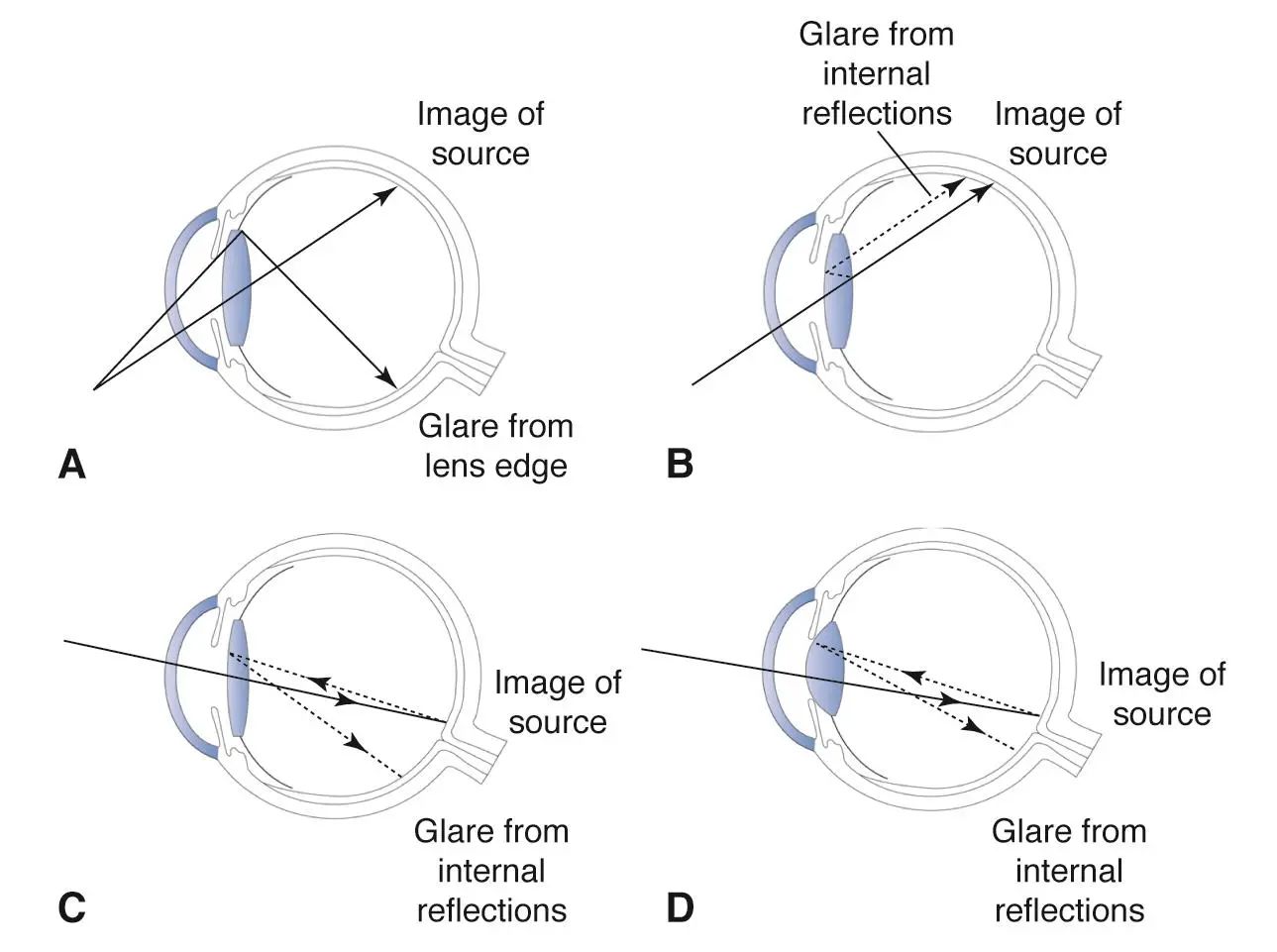
The leading hypotheses as to the cause of PD include the shape, size, index of refraction (I/R) and material makeup of the intraocular lens (IOL).[2] The truncated square edges of some PCIOL’s have been implicated as a causal factor for PD. Using reflectometry and ray tracing, Masket et al demonstrated that the obliquely oriented light ray striking the square edges reflect on to the retina and induce the symptoms of PD [3](Figure 1). They only occur when an external light source is present, especially when it is located obliquely at approximately 35 degrees. In support of this, Holladay et al has shown that square edges concentrate stray light into an arc on the retina opposite to the image of the light source.[4] On the other hand, rounded edge IOLs disperse stray light over a greater area of the retina, resulting in less PD symptoms.[5] Some changes in manufacturing of IOL’s have resulted in rounding of the anterior square edge, which may lower the incidence of PD symptoms. A high index of refraction has also been implicated as a causal factor for the development of PD.[5] ND seems to be multifactorial and is not as clearly understood as PD. ND occurs more frequently in women, in the left eye and with in-the-bag IOLs. The leading hypothesis describes the focal cause as an “illumination gap” between the light rays that are refracted by the IOL and those that miss the lenses optic and go to one part of the periphery [6] [7] [8] [9](Figure 2). Multiple studies suggest ND symptoms are more prevalent with thick rather than thin IOLs.[2] The cause of ND has evolved over the last decade. Masket et al positioned the optic anterior to the capsulotomy in reverse optic capture fashion thus causing anterior shifting of the illumination gap and theoretically ameliorating ND. Additional clinical associations have been seen with acrylic IOLs as well as high positive angle kappa values and high I/R. Horizontal inferotemporal haptic orientation may also minimize ND.[2] While no postoperative significance exists past day 1, optical modeling evaluation suggests the haptic junction illuminates the peripheral retina differently than the peripheral optic moving the illumination gap anteriorly. Additionally, there may be a central nervous system component as well, as Masket et al reported 80% reduction in the ND scotoma when the contralateral eye is occluded.[10] [11]
Some experience suggests postoperative capsular contraction may result in subtle bending/bowing of the implant lens (typically in the horizontal meridian, resulting in vertical axis, induced ATR astigmatism) and prove responsible for some of these complaints. Thinner implants being particularly susceptible to the relatively limited forces generated by the capsule's contraction. Overt capsulotomy phimosis being absent, there may at first be little suspicion, but prominent fibrosis of the anterior capsulotomy rim may be apparent on dilated inspection. Posterior capsulotomy in such a setting may increase, rather than relieve symptoms. Optic bending can produce mixed and often peripheral constructive and destructive interference patterns, which may explain some aspects of PD and ND symptoms. This model underscores benefits obtained with several surgical interventions: horizontal haptic placement, most immediately counteracting horizontal meridian contraction forces; sulcus placement, reverse optic capture and addition of piggyback lenses directly alleviating contractile force application and/or reinforcing the implant against bending; substitution of "unbendable" PMMA or thicker silicone implants, etc. Alleviation of symptoms may in these cases be achieved by multiple anterior capsular margin YAG relaxing incisions, perhaps as necessary carried to near the edge of the implant. One strong hint for this etiology is finding otherwise unexplained substantial discrepancy between the corneal cylinder magnitude/axis vs manifest refraction cylinder magnitude/axis in the postoperative eye. Even apparently "innocent" reduction from expected WTR cylinder, for instance, may suggest latent internal ATR cylinder from implant deformation is counteracting the corneal contribution.
Risk Factors
The primary risk factor for developing either type of dysphotopsia is cataract surgery. Risk factors for PD include IOL material and design such as truncated-edge IOLs (including both square and oval lenses) and ones with high I/R and high surface reflectivity. Risk factors for ND include small pupil, large angle kappa values, sharp IOL edge design, acrylic material, diameter, decentration, tilt, and aspheric surface.[12][13]
Primary Prevention
There is no definitive prevention method against postoperative dysphotopsia, however certain measures can be taken to reduce occurrence, especially for a second eye surgery when dysphotopsia occurred following the first. Use of a 6mm optic, reverse optic capture with positioning of the optic anterior to capsulorhexis, and horizontal orientation of haptics may minimize temporal light scatter. When operating on the second eye of a patient experiencing dysphotopsia, lens choice is important and should focus on a 3-piece silicone IOL with round edges, low chord mu value, and haptics positioned horizontally when able.[14] Despite these efforts, dysphotopsia could still occur in the second eye, so extensive counseling and discussion should be completed prior to surgery.
Diagnosis
History
Eliciting a good history is crucial in the diagnosis of dysphotopsia. Following CE/IOL, knowledge of the specific IOL manufacturer and lens type is important. Additionally, a detailed subjective examination includes any patient experience of bright artifacts of light described as arcs, streaks, starbursts, rings, or halos. Experiences of dark, temporal arcing shadows or curtains should also be discussed. If the patient is experiencing any of the following symptoms, attention to detail should focus on onset, location of the dysphotopsia in the visual field, characteristics, precipitating/alleviating factors, and severity of impairment on visual quality and activities of daily living. Reassurance and validation of the patient's symptoms are important.
Physical Examination
Patients with dysphotopsia may have a higher incidence of pseudophacodonesis. Pseudophacodonesis can be identified by visualizing the 4th Purkinje image at the slit lamp and examining for subtle movement. This is often best best seen without dilation.
Symptoms
Positive dysphotopsia typically presents with bright artifacts of light described as arcs, streaks, starbursts, rings, or halos. Negative dysphotopsia typically presents as dark, temporal arcing shadows or curtains.[2]
Clinical Diagnosis
Clinicians rely primarily on patient-reported outcomes and symptom description for clinical diagnosis, which is the primary means of diagnosing dysphotopsia.
Diagnostic procedures
Even though ND can sometimes be accompanied by far peripheral visual field changes on Goldmann kinetic perimetry, no consistently reliable objective tests exist to examine the presence of either PD or ND.[2]
Differential diagnosis
- Retinal detachment
- Posterior capsule opacification[15]
- Posterior capsule striae causing Maddox rod effect
- Posterior vitreous detachment
- Entoptic phenomenon
Management
Medical therapy
Conservative management is the primary nonsurgical approach as most of these symptoms diminish within a matter of weeks. Pharmacologic miosis with drops such as pilocarpine and brimonidine can help relieve PD, especially at nighttime, but does little for ND symptoms. Spectacles with a thick frame, even if not visually necessary, can help with ND as the brain attributes the shadow to the frames. Additionally, management should address other confounding visual complaints through correction of any refractive error, treatment of any coexisting ocular surface disease such as dry eye, and cautious treatment of PCO if IOL exchange is not required or expected in the future.[2]
Lens manufacturing companies have addressed PD by making the anterior portion of the square edge rounder, reducing the thickness of the IOL square edge, leaving the IOL edge unpolished, and moving the IOL optical power more anterior rather than posterior. PD remains a significant postoperative problem despite these changes.[2]
Surgery
Surgical options should be evaluated on a case specific basis. One mainstay option to reduce PD includes using IOL materials with a lower index of refraction, low chord mu value, reduced surface reflectivity, and rounded edges.[3] This is especially important in the second eye of patients who are experiencing dysphotopsia from previous IOL. Intraocular lens exchange to polymethyl methacrylate (PMMA), silicone, or copolymer IOLs in the capsular bag or ciliary sulcus has also been reported to be mostly successful.[3] Reverse optic capture, both primary as prophylaxis and secondary as treatment, has also been shown to ameliorate dysphotopsia, primarily ND as the light shifts anteriorly decreasing the illumination gap.[16] Additionally, implementation of piggyback or sulcus-placed add-on IOLs has been shown to improve ND. Optical modeling evaluation also suggests that orienting the haptic horizontally may decrease ND, though there has been no statistical significance after postoperative day 1. Limited success has been reported after Nd:YAG capsulotomy of the nasal anterior capsule.[16]
Prognosis
While most patient experiences of dysphotopsia resolve or undergo neuroadaptation, persistent symptoms may still be present despite lens characteristics and surgical correction. Persistent ND symptoms occur in approximately 3% of patients at 1 year after surgery[17], while up to 49% of patients experience PD symptoms with up to a 76-88% improvement in PD symptoms after changing IOL material.[2] Prognosis is favorable regardless of PD or ND without significant visual impairment.
References
- ↑ Lens-related vision disturbances. American Academy of Ophthalmology. https://www.aao.org/education/image/lens-related-vision-disturbances Accessed July 6, 2023.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Masket S, Fram NR. Pseudophakic Dysphotopsia: Review of Incidence, Cause, and Treatment of Positive and Negative Dysphotopsia. Ophthalmology. 2020 Aug 12:S0161-6420(20)30787-9. doi: 10.1016/j.ophtha.2020.08.009. Epub ahead of print. PMID: 32800744.
- ↑ Jump up to: 3.0 3.1 3.2 Masket S, Rupnick Z, Fram NR, et al. Surgical Management of Positive Dysphotopsia: US Perspective [published online ahead of print, 2020 Jul 13]. J Cataract Refract Surg. 2020. https://doi.org/10.1097/j.jcrs.0000000000000307.
- ↑ Holladay JT, Bishop JE, Lewis JW. Diagnosis and treatment of mysterious light streaks seen by patients following extracapsular cataract extraction. J Am Intraocul Implant Soc. 1985;11:21e23.
- ↑ Jump up to: 5.0 5.1 Erie JC, Bandhauer MH, McLaren JW. Analysis of post- operative glare and intraocular lens design. J Cataract Refract Surg. 2001;27(4):614e621
- ↑ Holladay JT, Simpson MJ. Negative dysphotopsia: causes and rationale for prevention and treatment. J Cataract Refract Surg. 2017;43:263e275
- ↑ Hong X, Liu Y, Karakelle M, et al. Ray-tracing optical modeling of negative dysphotopsia. J Biomed Opt. 2011;16(12): 125001e12500
- ↑ Simpson MJ. Double image in far peripheral vision of pseudophakic eye as source of negative dysphotopsia. J Opt Soc Am A Opt Image Sci Vis. 2014;31:2642e2649
- ↑ Erie JC, Simpson MJ, Bandhauer MH. Effect of a sulcus-fixated piggyback intraocular lens on negative dysphotopsia: ray-tracing analysis. J Cataract Refract Surg. 2019;45(4): 443e449.
- ↑ Masket S, Rupnik Z, Fram NR. Neuroadaptive changes in negative dysphotopsia during contralateral eye occlusion. J Cataract Refract Surg. 2019;45:242e243.
- ↑ Masket S, Rupnik Z, Fram N, Vikesland R. Binocular Goldmann Visual Field Testing of Negative Dysphotopsia. J Cataract Refract Surg. 2020;46(1):147e148.
- ↑ Holladay JT, Zhao H, Reisin CR. Negative dysphotopsia: the enigmatic penumbra. J Cataract Refract Surg. 2012 Jul;38(7):1251-65. doi: 10.1016/j.jcrs.2012.01.032. PMID: 22727295.
- ↑ Stephenson M. Dysphotopsia: Not Just Black and White. Review of Ophthalmology. 2017 Nov.
- ↑ Makhotkina NY, Berendschot TT, Nuijts RM. Objective evaluation of negative dysphotopsia with Goldmann kinetic perimetry. J Cataract Refract Surg. 2016;42:1626–1633.
- ↑ Nishi O. Posterior capsule opacification. Part 1: experimental investigations. J Cataract Refract Surg. 1999;25:106e117
- ↑ Jump up to: 16.0 16.1 Masket S, Fram NR, Cho A, Park I, Pham D. Surgical management of negative dysphotopsia. J Cataract Refract Surg. 2018 Jan;44(1):6-16. doi: 10.1016/j.jcrs.2017.10.038. PMID: 29502619.
- ↑ Osher RH. Negative dysphotopsia: long-term study and possible explanation for transient symptoms. J Cataract Refract Surg. 2008;34:1699e1707.