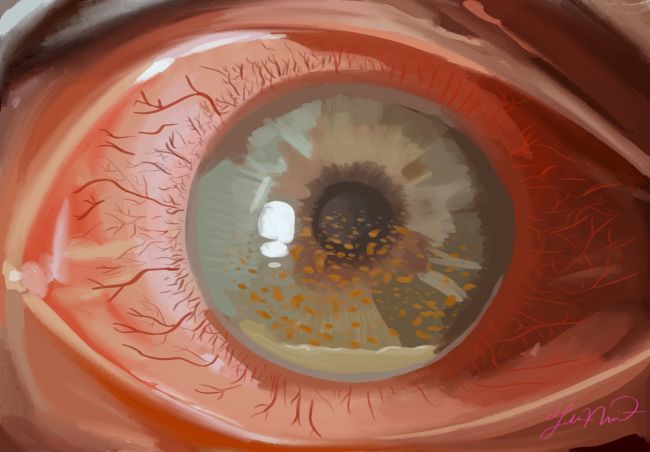
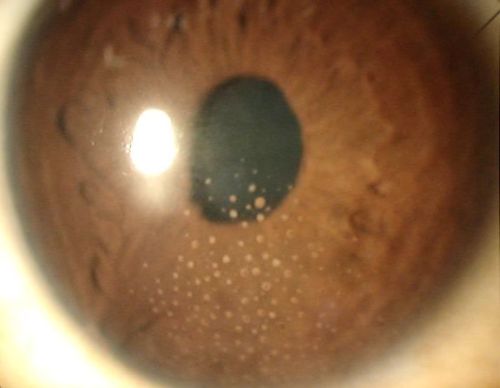
Cytomegalovirus Anterior Uveitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Uveitis refers to a spectrum of intraocular inflammatory diseases which are caused by both infectious and non-infectious etiologies. Uveitides are differentiated clinically and histologically based on the type of inflammation (granulomatous or non-granulomatous), anatomic location (anterior segment, posterior segment or both) as well as the pattern and duration of inflammation (acute, chronic or recurrent). This review will focus on cytomegalovirus anterior uveitis (CMV AU), which is a viral infection characterized by inflammation of the anterior uveal tract (i.e. iris and ciliary body). CMV AU is the most common ocular manifestation of CMV disease in immunocompetent individuals and often presents as unilateral blurred vision, eye pain, increased intraocular pressure (IOP) and conjunctival injection. [1][2]
ICD-10-CM Coding Diagnosis: B25.9 and H20.9
Etiology
CMV AU is caused by cytomegalovirus, which is a DNA virus that is a member of the Herpesviridae family. Primary CMV infection is common, with 80 to 85% seropositivity rate in adults aged ≥ 40 years.[3] In immunocompromised hosts, the virus causes necrotizing retinitis. Until recently CMV has not been thought to cause any diseases in immunocompetent hosts. Like other Herpesviridae (e.g. Herpes Simplex Virus (HSV), Varicella-Zoster Virus (VZV), and Epstein-Barr Virus) CMV evades immune surveillance and establishes latency. Because CMV lies latent in myeloid progenitor cells, it is thought that CMV AU is due to viral reactivation in anterior chamber monocytes in patients who are immunosuppressed and mounting a systemic immune response. [1][2][4]
Risk factors
Although there have been reports of CMV anterior uveitis from across the world, most reported cases come from Asian countries, mainly involving Chinese and Japanese populations. While the exact reason for this in still an area of research, it is important to note that the seroprevalence of CMV is higher in Asia than in the west. Some suggested reasons for this geographic disparity include genetic susceptibilities and difference in viral virulence. Two common forms of CMV anterior uveitis have been shown to have a bimodal presentation age. For example, recurrent acute anterior uveitis typically presents in patients in their third to fifth decade, while chronic hypertensive anterior uveitis tends to present in older patients in the fifth to seventh decade. Regardless of race and age, CMV anterior uveitis has been shown to predominantly affect men. [2]
General pathology
CMV anterior uveitis is a result of reactivated cytomegalovirus, which is a DNA virus that is a member of the Herpesviridae family. [1]
Pathophysiology
CMV infection is common, and the virus can remain latent in mononuclear cells. Unless the immune system is compromised, CMV is usually suppressed. For reasons that are not completely understood, infections that are ineffectively controlled can cause local inflammation and reactivation of CMV that is latent in macrophage and dendritic cells in the anterior segment. It is also possible that another infection at another location can lead to CMV reactivation in circulating monocytes. Reactivation results in cytokine release (e.g. interferon-γ or interferon-β) that causes tissue inflammation. [2]
Primary prevention
Primary prevention of CMV anterior uveitis includes lifestyle modifications such as appropriate diet, exercise, and control of other chronic medical conditions such as diabetes and hypertension. There is currently not a vaccine available for CMV.
Diagnosis
History
CMV AU has a variety of ocular manifestations. It typically presents in immunocompetent patients as unilateral blurred vision, eye pain, and conjunctival injection; patients may also experience visual halos.[1] Most presentations of CMV AU have a more insidious onset and chronic course than HSV or VZV, which typically present more acutely.[5] CMV AU can present similarly to Posner-Schlossman Syndrome (PSS) as a recurrent acute hypertensive anterior uveitis or similar to Fuchs uveitis syndrome (FUS) as a chronic hypertensive anterior uveitis with anterior segment features resembling that of Fuchs uveitis syndrome (FUS).[2] There are also multiple case reports of CMV AU following immunosuppressant drugs like dexamethasone, topical cyclosporine, and prostaglandin inhibitors. Because these medications are widely used for common ocular disorders such as dry eye, it is important that ophthalmologist be aware that latent CMV infection has the potential to reactivate and present as anterior uveitis following their use. [6]
Physical exam
- Best corrected visual acuity
- External examination of eyelids and periocular skin.
- Measurement of intraocular pressure
- Slit-lamp biomicroscopy of the anterior segment with special attention to any staining corneal defects or plaques, stromal opacities, corneal vascularization, iris atrophy, iris heterochromia, keratic precipitates, and anterior chamber inflammation.
- Dilated examination of the lens, macula, peripheral retina, optic nerve, and vitreous – imperative on every uveitis patient.
Signs
CMV AU has a spectrum of clinical manifestations including endotheliitis, iris atrophy, and anterior uveitis which may present in an acute form as Posner-Schlossman syndrome (PSS) or more chronically as Fuchs uveitis syndrome (FUS).
PSS is a unilateral uveitis that predominantly affects younger men aged 20–50 years. In PSS patients, , it isthe conjunctiva is minimally injected and if there is typical that the eye is minimally injected, and that significantly elevated IOP between 40 and 60 mmHg, typically leads to corneal epithelial edema will develop. [7]Additional signs of PSS include its relapsing and remitting course, its relatively mild anterior chamber inflammation, and characteristic grey-white medium-to-large granulomatous keratic precipitates (KPs). In contrast, FUS is a chronic anterior uveitis of insidious onset that is more typical in older patients in the fifth to seventh decade40s to 60s. These patients present with mild injection, moderate anterior chamber inflammation and diffuse, fine, stellate KPs distributed evenly over the endothelium.[2] In each instance, patients can develop iris atrophy, likely secondary to direct invasion of the iris stroma by virus or by ischemic necrosis related to extreme fluctuation in IOP.[1] Other signs include corneal endotheliitis and late onset of cataracts.[2]
Symptoms
CMV AU typically presents with unilateral eye pain, conjunctival injection, and blurring of vision that may be associated with halos and ipsilateral headache. [2]
Clinical diagnosis
The diagnosis of CMV AU can be difficult because other more common infectious causes of anterior uveitis such as HSV (HSVC AU) and VZV (VZV AU) often have overlapping clinical signs.[1] General clinical features associated with CMV anterior uveitis include elevated IOP, anterior chamber inflammation, iris atrophy, endotheliitis and, importantly, characteristic keratic precipitates (KPs).[8] Although morphologies of KPs can vary in appearance and distribution, specific KP have associations with the PSS and FUS presentations of CMV anterior uveitis as previously described.[9] Moreover, it has been reported that circinate KPs (or “KP corrals”) are highly suggestive of CMV ocular infection generally and can help distinguish CMV from other types of herpetic anterior uveitis.[8]
Hyperemia and pain of the eye, blurring of vision, ciliary injection, medium-to-large KPs, cells and flare in the anterior chamber, and posterior synechia have been reported as being significantly greater in HSV AU and VZV AU vs CMV AU. In contrast, small KPs, diffuse iris atrophy, elevated IOP, and glaucoma surgery were significantly greater in CMV AU versuss HSV AU and VZV AU.[10] In many cases, patients are diagnosed after having been unsuccessfully treated for chronic herpetic uveitis with acyclovir and topical steroids. [1]
Diagnostic procedures
Although most anterior uveitis case are non-infectious, viral etiologies represent an estimated 10% of anterior uveitis cases.[11] If infectious anterior uveitis is suspected, anterior chamber paracentesis should be performed to obtain aqueous fluid, which can be analyzed to differentiate among CMV, HSV, and VZV in several different ways.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR):
Standard CMV nucleic acid amplification with real-time polymerase chain reaction (RT-PCR) can detect viral genome load in the aqueous humor. The PCR needed for CMV (a DNA virus) should be real time (quantitative) because low levels of CMV can be found in any type of anterior segment inflammation (as it is an intracellular virus). No standard has been set for the number of copies required to call CMV versus bystander effect, but Kandori et al. reported that > 103 copies/mL of CMV in the aqueous were associated with elevated intraocular pressure (IOP), corneal endothelial cell loss, and recurrent inflammatory episodes. Although RT-PCR has good sensitivity (previously reported at 71%) in acute phase uveitis, it is reportedly less effective at confirming diagnosis in chronic cases marked by low grade inflammation.[11] In these chronic cases, clinical diagnosis is more supported by antibody detection in the anterior chamber fluid.
Anterior Chamber Antibody test:
One such test, theThe Goldmann-Witmer coefficient (GWc) compares levels of intraocular antibody to antibody loads in the serum. Recently, a more specific CMV driven intraocular antibody synthesis analysis termed the intraocular antibody index (AI) assay has been proposed, with improved sensitivity (87%).
Ultimately, RT-PCR and antibody analysis are complimentary for accurately diagnosing CMV anterior uveitis and in combination can achieve 100% sensitivity in one single aqueous tap.[12]
Differential diagnosis
Non-CMV herpetic anterior uveitis
CMV anterior uveitis is relatively uncommon when compared to HSV and VZV and therefore often remains low on the differential unless a patient fails treatment with acyclovir or valacyclovir. In addition to infectious herpetic anterior uveitis, it is important to work up other possible non-infectious etiologies. [1]
Idiopathic hypertensive anterior uveitis
Because of reported incidence of CMV presenting as Posner–Schlossman syndrome or Fuchs uveitis syndrome it is important to rule out CMV infection in these scenarios, particularly in Asia. [1]
Endothelial rejection post corneal graft
In eyes with previous grafts, the KPs associated with CMV anterior uveitis occurred on both the donor and host corneas unlike in endothelial rejection, where the KPs are seen on the donor.[1]
Fuchs Heterochromic Iridocyclitis
Although CMV anterior uveitis can present in FUS-like chronic anterior uveitis, distinguishing FUS from FUS-like CMV anterior uveitis is important for choosing the right treatment. Endotheliitis, endothelial cell loss, nodular endothelial lesions, that can also be “coin-shaped” and with surrounding halo are suggestive to CMV anterior uveitis. Also, the iris atrophy pattern is different for FUS and FUS-like CMV anterior uveitis. The “patchy” iris atrophy, low transillumination and rare heterochromia are typical for CMV anterior uveitis.[13]
Management
General treatment
Early initiation of therapy is critical in the management of CMV AU due to its association with sight threatening complications. The general treatment of CMV AU includes antiviral drugs, IOP control, and corticosteroids, if needed. Ganciclovir or the oral prodrug valganciclovir is the current treatment of choice for CMV anterior uveitis. Ganciclovir is a potent inhibitor of herpes viruses, including CMV. It is a nucleoside analogue that suppresses the replication of herpes viruses. CMV is not sensitive to acyclovir, valacyclovir, or penciclovir, and inadequate response to this medication can be a sign of CMV infection.
Ocular hypertension resulting from CMV anterior uveitis can usually be managed with glaucoma medications, which typically includes the initiation of beta-blockers, alpha-agonists, and carbonic anhydrase inhibitors in a stepwise fashion. The prescription of prostaglandin analogues is avoided in CMV uveitis because of concerns that they may worsen herpetic infections. Topical corticosteroids and anti-glaucoma medications can be weaned as inflammation and elevations in eye pressure subside. [1][14]
Medical Therapy
There are different formulations of ganciclovir including oral, topical gel, intravenous, implantable, or intravitreal. Recently, it has been reported from North American tertiary centers that oral valganciclovir in combination with as needed topical corticosteroids is effective at acutely controlling inflammation, maintaining a durable quiescence at 6 months and one year, and reducing incidence of elevated IOP. Patients were begun on a loading dose of 900 mg BID for a period of at least two weeks and were subsequently decreased to maintenance dose of 450 mg BID after achieving inactive inflammation clinically. The drug was generally well tolerated and safe, although routine laboratory monitoring for blood counts is needed. It is important to note that disease recurrence up to 80% has been reported if the dose is decrease or discontinued, suggesting that long term prophylactic therapy is necessary.
An alternative to oral valganciclovir that has been used to treat CMV anterior uveitis is 0.15% topical ganciclovir. The benefits of local therapy include decreased systemic toxicity, which obviates the need for frequent laboratory monitoring. It has been reported that although topical ganciclovir may be beneficial in reducing the frequency of recurrence in patients with CMV anterior uveitis, it is not statistically associated with prolonging the time-to-recurrence. The Systemic and Topical Antiviral Control of CMVAU: Treatment Outcomes (STACCATO) randomized control trial seeks to compare these 2 treatment modalities along with placebo therapy in achieving quiescence during an acute flare and in preventing long-term recurrences (clinicaltrials.gov registration: NCT03576898). [14][8]
Medical Follow up
During treatment with oral ganciclovir, it is important to review evaluate patients at weekly intervals for the first month and then at monthly intervals, with more frequent reviews as necessary. Follow up visits should assccess the patient’s best-corrected visual acuity, IOP and anterior chamber inflammation. Complete blood count and serum creatinine levels should be monitored every 2 weeks in patients receiving longer courses of the systemic antiviral treatment. It is also important to consult an infectious diseases physician who can also review the patient regularly. [15][8]
Surgery
As previously described glaucoma is an important complication of CMV anterior uveitis, and an estimated one fourth of patients require glaucoma filtration surgery within 4 years of being diagnosed. Moreover, if intraocular inflammation and steroid use causes a cataract then cataract surgery may be performed when the infection is adequately controlled. [7]
Surgical follow up
Depending on the type of surgery performed, the patient should be closely monitored at regular post-operative visits for severe inflammation. Viral prophylaxis with antiviral therapy and steroids should be strongly considered.
Complications
CMV AU can result in significant visual morbidity deterioration due to its recurrent or chronic disease course. The main complications include secondary open and steroid induced glaucoma, cataract formation, and corneal decompensation.[14] The longer the delay in diagnosis, inappropriately beginning long-term steroid therapy (which provokes CMV reactivation) and uncontrolled intraocular pressure all increase the risk of complications. [16]One long term French series reported that more than one-fourth of patient required a glaucoma surgery within 4 years. End-stage glaucoma can also cause irreversible corneal edema that requires corneal transplantation. [17]
Prognosis
Prognosis is greatly variable and dependent on long-term sequelae. Long-term vision loss, need for surgery, and long-term antiviral prophylaxis are all possible.
Additional Resources
- CDC CMV Resource Center
- CDC Information on Varicella Vaccination
- CDC Information on Shingles Vaccination
- Elia M, Huang JJ, Gaudio PA, Fekrat S, Scott IU. Cytomegalovirus Anterior Uveitis in Immunocompetent Patients. Ophthalmic Pearls. EyeNet Magazine. San Francisco: American Academy of Ophthalmology, 2016.
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Maxwell Elia, M., John J. Huang, MD, and Paul A. Gaudio, MD. (2016). Cytomegalovirus Anterior Uveitis in Immunocompetent Patients. American Academy of Ophthalmology EyeNet Magazine. Retrieved from https://www.aao.org/eyenet/article/cytomegalovirus-anterior-uveitis-in-immunocompeten
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Chan, N. S., Chee, S. P., Caspers, L., & Bodaghi, B. (2018). Clinical Features of CMV-Associated Anterior Uveitis. Ocul Immunol Inflamm, 26(1), 107-115. doi:10.1080/09273948.2017.1394471
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4659396/
- ↑ Wong, J. X., Agrawal, R., Wong, E. P., & Teoh, S. C. (2016). Efficacy and safety of topical ganciclovir in the management of cytomegalovirus (CMV)-related anterior uveitis. J Ophthalmic Inflamm Infect, 6(1), 10. doi:10.1186/s12348-016-0078-z
- ↑ Terada, Y., Kaburaki, T., Takase, H., Goto, H., Nakano, S., Inoue, Y., . . . Mochizuki, M. (2021). Distinguishing Features of Anterior Uveitis Caused by Herpes Simplex Virus, Varicella-Zoster Virus, and Cytomegalovirus. Am J Ophthalmol, 227, 191-200. doi:10.1016/j.ajo.2021.03.020
- ↑ Siak, J., & Chee, S. P. (2018). Cytomegalovirus Anterior Uveitis Following Topical Cyclosporine A. Ocul Immunol Inflamm, 26(1), 90-93. doi:10.1080/09273948.2017.1306083
- ↑ Jump up to: 7.0 7.1 Jap, A., & Chee, S.-P. (2014). Cytomegalovirus-associated anterior segment infection. Expert Review of Ophthalmology, 6(5), 517-528. doi:10.1586/eop.11.49
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Bhoopat, T., Takhar, J. S., Oldenburg, C. E., Keenan, J. D., Gonzales, J. A., & Margolis, T. P. (2020). Treatment of Cytomegalovirus Anterior Uveitis at a North American Tertiary Center With Oral Valganciclovir. Cornea, 39(5), 584-589. doi:10.1097/ICO.0000000000002251
- ↑ Park, S. W., & Yu, H. G. (2013). Association of cytomegalovirus with idiopathic chronic anterior uveitis with ocular hypertension in Korean patients. Ocul Immunol Inflamm, 21(3), 192-196. doi:10.3109/09273948.2012.754908
- ↑ Terada, Y., Kaburaki, T., Takase, H., Goto, H., Nakano, S., Inoue, Y., . . . Mochizuki, M. (2021). Distinguishing Features of Anterior Uveitis Caused by Herpes Simplex Virus, Varicella-Zoster Virus, and Cytomegalovirus. Am J Ophthalmol, 227, 191-200. doi:10.1016/j.ajo.2021.03.020
- ↑ Jump up to: 11.0 11.1 Relvas, L. J. M., Antoun, J., de Groot-Mijnes, J. D. F., Motulsky, E., Ten Dam-Van Loon, N. H., Makhoul, D., . . . Caspers, L. (2018). Diagnosis of Cytomegalovirus Anterior Uveitis in Two European Referral Centers. Ocul Immunol Inflamm, 26(1), 116-121. doi:10.1080/09273948.2017.1411952
- ↑ De Simone, L., Belloni, L., Aldigeri, R., Zerbini, A., Mastrofilippo, V., Sangiovanni, A., . . . Cimino, L. (2019). Aqueous tap and rapid diagnosis of cytomegalovirus anterior uveitis: the Reggio Emilia experience. Graefes Arch Clin Exp Ophthalmol, 257(1), 181-186. doi:10.1007/s00417-018-4180-9
- ↑ Classification Criteria for Cytomegalovirus Anterior Uveitis. (2021). American Journal of Ophthalmology, 228, 89–95. doi:10.1016/j.ajo.2021.03.060
- ↑ Jump up to: 14.0 14.1 14.2 Wong, J. X., Agrawal, R., Wong, E. P., & Teoh, S. C. (2016). Efficacy and safety of topical ganciclovir in the management of cytomegalovirus (CMV)-related anterior uveitis. J Ophthalmic Inflamm Infect, 6(1), 10. doi:10.1186/s12348-016-0078-z
- ↑ Chee, S. P., & Jap, A. (2010). Cytomegalovirus anterior uveitis: outcome of treatment. Br J Ophthalmol, 94(12), 1648-1652. doi:10.1136/bjo.2009.167767
- ↑ Touhami, S., Qu, L., Angi, M., Bojanova, M., Touitou, V., Lehoang, P., . . . Bodaghi, B. (2018). Cytomegalovirus Anterior Uveitis: Clinical Characteristics and Long-term Outcomes in a French Series. Am J Ophthalmol, 194, 134-142. doi:10.1016/j.ajo.2018.07.021
- ↑ Kam, K. W., Wong, C. H., Ho, M., Sze, R. K. H., Chan, P. K. S., & Young, A. L. (2021). Iris Depigmentation in the Prediction of Cytomegalovirus Anterior Uveitis. Ocul Immunol Inflamm, 1-6. doi:10.1080/09273948.2021.1952277