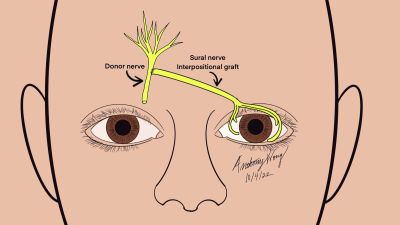
Corneal Neurotization for Neurotrophic Keratitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Video 1. Minimally Invasive Transblepharoplasty Corneal Neurotization With Ipsilateral Supraorbital Nerve. This video highlights the surgical steps of corneal neurotization via a minimally invasive blepharoplasty incision. (Video Made by Anthony S. Wong, M.D., Surgeon: Paul Phelps, MD, FACS)
Background
Neurotization refers to the technique of relocating a healthy nerve or its proximal stump to reestablish an impaired sensory or motor pathway that has sustained irreparable damage. Corneal Neurotization is the process of re-innervating the cornea by way of nerve transfer. It may be achieved via direct local nerve transfer or indirectly with an interpositional nerve graft. Methods to restore sensitivity to the cornea via neurotization is of particular importance because sensation to the cornea is requisite for the blink reflex, adequate tear production, and proper corneal epithelial homeostasis. Corneal hypoesthesia or anesthesia can be devastating, leading to a persistently vulnerable state as there is loss of protection, epithelial cell regeneration and migration, and loss of trophic elements to the cornea. Corneal neurotization serves as a durable and effective method to remedy neurotrophic keratitis.
Corneal Anatomy
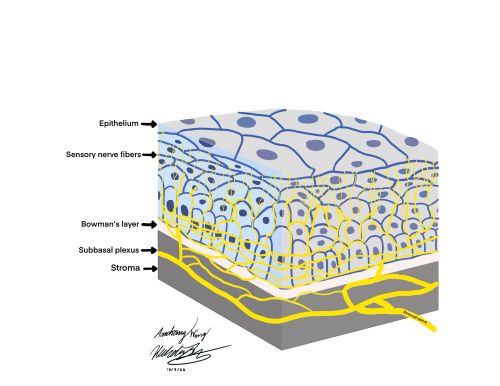
As the optically transparent, avascular, and most anterior portion of the eye, the cornea is responsible for two thirds of the eye’s total refractive power. The cornea is one of the most densely innervated structures in the body with approximately 7,000 nociceptors per square millimeter, giving rise to sensitivities 40 times greater than tooth pulp and 400-600 times greater than skin. [1][2] Innervation to the cornea originates from the first division (V1 - ophthalmic branch) of the trigeminal nerve, distally traveling in the nasociliary nerve and long ciliary nerve branches and ultimately dividing into fibers that invade the stroma [3]. These nerve branches then run parallel to the layers of the cornea between Bowman’s layer and the basal epithelium to form a nerve plexus known as the subbasal nerve plexus.
Role of Corneal Nerves
The nerves supplying the cornea are vital for its appropriate function. Not only do they assist in protecting the cornea from external and mechanical insults like dust and debris, but also coordinate communication between the blink reflex, lacrimal secretion, and maintaining the tear film. A properly lubricated ocular surface is paramount for both ensuring optimal visual acuity and for maintaining structural integrity of the epithelial barrier.
On a microscopic level, the corneal nerves play an essential role in corneal homeostasis. The corneal nerves contain and release neurotransmitters responsible for epithelial healing and upkeep like substance P, calcitonin gene-related peptide (CGRP), neuropeptide Y, and vasoactive intestinal peptide. [4]
The nerves also release trophic components like neurotrophin 3 (NT-3), neurotrophin 4 / 5 (NT-4), ciliary neurotrophic factor, brain-derived neurotrophic factor, and glial cell derived neurotrophic factor. [4] Collectively, these growth-stimulating factors all play roles to assist with epithelial cell proliferation, migration, regeneration, and viability.
Corneal Nerve Fiber Types
The intricate nerve supply to the cornea allows for transmission of multiple input types such as mechanical, chemical, and thermal stimuli. The distribution of corneal nociceptor types are as follows :[5]
| Composition | Type of Receptor | Interpretation | Input | Fiber Type |
|---|---|---|---|---|
| 20% | Mechanoreceptors | Acute/ Sharp Pain | Mechanical Input | Thin Myelinated Aδ nerves |
| 70% | Polymodal | Sustained pain/ Sharp | Chemical stimuli (Ach, prostaglandins, bradykinin), irritants, also heat and mechanical stimuli | Slow, unmyelinated C type nerves |
| 10% | Cold receptors | Dryness, air | Tear film evaporation, cold stimuli, air | Aδ and C fiber cold receptors |
Pathogenesis
Corneal Anesthesia/Neurotrophic Keratitis
As aforementioned, proper corneal sensation is crucial to the proper maintenance, growth, defense, and healing of the corneal epithelium. When sensory innervation to the cornea is diminished or absent, neurotrophic keratitis can occur. Neurotrophic keratitis refers to the loss of corneal nerve function and sensation from a degenerative disease. With a prevalence of less than 5 in 10,000, neurotrophic keratitis is a degenerative epitheliopathy that can lead to significant morbidity.[6]
When loss of corneal sensation occurs, the balance between reflexive blinking and tear production is lost. This leaves the corneal epithelial surface vulnerable and prone to mechanical abrasion and epithelial barrier erosion and breakdown. Additionally, nerve dysfunction causes a decrease in release of important cell turnover mediators like substance P, multiple neurotrophic factors, calcitonin gene-related peptide, neuropeptide Y. [7]Therefore, the insult to homeostasis is bipartite: there is increased mechanical degradation of the epithelium and there is decreased regenerative capacity of the epithelial cells.
Ultimately, profound morbidity from neurotrophic keratitis presents in the form of recurrent epithelial defects, corneal ulcers, corneal perforation, and frequent need for treatment.
Causes
Corneal hypoesthesia and anesthesia primarily arises from injury to the nerves supplying the cornea. The leading attributable causes are viral infection from herpetic keratitis (27-32% from herpes simplex keratoconjunctivitis and herpes zoster keratoconjunctivitis) followed by injury to the cornea like chemical burns, iatrogenic damage after surgery, and accidental trauma. [8][9]
More proximally, the trigeminal nerve and ganglion can sustain compressive damage from space-occupying lesions like neuromas, meningiomas, and aneurysms, leading to dysfunctional transduction of signals, and subsequently decreased corneal sensation. Corneal disease like keratoconus and ocular surface diseases like keratoconjunctivitis sicca, and contact lens overuse can also contribute to corneal anesthesia.
Medications like topical anesthetics, trifluridine, beta blockers, and diclofenac sodium, as well as preservatives, such as benzalkonium chloride (BAK), have a role in disrupting corneal sensitivity. Congenital diseases like Riley Day syndrome, Moebius corneal hypoesthesia, and Goldenhar-Gorlin syndrome also are potential causes of corneal anesthesia. [9]Systemic diseases, namely diabetes, vitamin A deficiency, leprosy, and multiple sclerosis, ultimately have a deleterious effect on nerves can lead to decreases in appropriate corneal sensory nerve function.[8]
Surgical History
Neurotization has been described since the early 1900’s and has been an established treatment modality for brachial plexus injuries and peripheral nerve injuries with sensory or motor loss. [10][11]Neurotization as a treatment modality for other conditions has recently been described as well, such as surgical treatment for facial nerve paralysis and sensation loss post breast reconstruction.[12]
Neurotization in the field of ophthalmology, namely corneal neurotization, however, has more recently taken the stage as a treatment modality. The notion of corneal neurotization was possibly first drafted by Samii in 1972 where he described connecting the greater occipital nerve to a transected proximal ophthalmic nerve using the sural nerve as an interpositional graft.[13] [6] The novel procedure outcome was met with minimal incorporation into practice due to its limited postoperative success, frontal craniotomy approach, and long procedure time. [6] [14]
In 2009, Terzis et al., presented the first corneal neurotization surgery that would serve as the basis for modern corneal neurotization surgeries. He described a direct corneal neurotization in cases of facial nerve palsy with ipsilateral trigeminal nerve involvement and corneal anesthesia. The approach consisted of transfer of the contralateral supratrochlear and supraorbital nerves directly to the corneoscleral limbus of a neurotrophic cornea, yielding substantial recovery of corneal sensation as assessed by the Cochet-Bonnet esthesiometer. [10] Since then, others have achieved similar results using variations of direct neurotization (contralateral supraorbital/supratrochlear, ipsilateral supraorbital/supratrochlear, endoscopic) and indirect neurotization, which usually involves coapting a sural nerve graft to the contralateral supraorbital or supratrochlear nerves. Newer grafts with the greater auricular nerve are being explored as well. [15][10][13][16]
Indications
The indication for corneal neurotization is for patients with neurotrophic keratitis with clinically significant and visually significant morbidity. Patients may experience persistent and recurrent punctate epithelial erosions, epithelial defects, ulceration, neovascularization, scarring, corneal melt and perforation, and denervation of at least 6 months.[17] Corneal neurotization may also be considered for patients with poor response to alternative medical management or in patients with inability to obtain or sustain medical treatment, and for patients with poor prognosis for long term improvement. Although a majority of patients undergoing the surgery in the literature are from traumatic loss of sensation, There is also an indication in post-herpectic neurotrophic keratopathy as well as congenital trigeminal anesthesia and aplasia in the setting of Goldenhar syndrome. [18][19][20]
Consideration of Alternatives
Alternative treatment modalities should be considered in management for a patient with neurotrophic keratitis. However, many of the standing treatment options serve as supportive measures rather than curative solutions. Options like lubrication involving artificial or serum tears, contact lenses, and amniotic membrane grafts can help with epithelial surface violations and inflammation. Surgical treatment options like tarsorrhaphy can also prove useful to treat corneal ulcers.
Other than these supportive measures, there are some medical treatments options that address the underlying cause of denervation like corneal neurotization. Topical recombinant human nerve growth factor is a recent development and an alternative treatment option for neurotrophic keratitis. In a clinical trial of patients with Mackie stage 2–3 neurotrophic corneas, there was an increased rate of complete resolution of epithelial defects and a decreased rate of recurrence after complete healing. However, improvement in corneal sensation at 48 weeks post-treatment was statistically insignificant. It is important to note; however, that longer-term studies are needed to elucidate the long term feasible use and long term improvement in sensation. [6]
Cenegermin (Oxervate™) is a recombinant human nerve growth factor (rhNGF), for the treatment of neurotrophic keratitis. In the U.S., it has been approved for use on pediatric patients, as well. The most common side effects of cenegermin include eye and eyelid pain, inflammation, increased tearing, and foreign body sensation in the eye during the treatment period. [21] It is important to note, however, that significant logistical and financial burden may exist in obtaining and administering the drug, especially in resource poor settings.
Substance P, a neurotransmitter that is released from corneal nerve fibers in response to inflammation, works in concert with Insulin-like Growth Factor to promote epithelial cell proliferation and corneal wound healing. This healing modality has shown potential in early neurotrophic keratitis, but has not yet produced promising evidence for advanced stage disease. [21]
Serum tears and other blood derivatives have a utility profile which includes growth factors and have been efficacious in epithelial healing. There has been proven mild improvement of corneal sensation in neurotrophic corneas, but the difficult access and risk for contamination stand as a barrier to their use. [6]
Thymosin beta 4 (Tβ4) is an endogenous polypeptide that facilitates healing by suppressing ocular inflammation and promoting corneal epithelial growth by decreasing levels of proinflammatory cytokines, such as TNF-α. A case series of Tβ4 applied to nine eyes with stage 2 NK and chronic nonhealing corneal epithelial defects demonstrated its potential as a medical alternative to corneal neurotization. A phase 3 randomized clinical study is underway to further determine the beneficial role of Tβ4. [21]
Regenerating Agents (RGTAs), Plasma Rich Growth Factors, Netrin 1, Coenzyme Q10, and amniotic membrane extract eye drops (AMEED) are other emerging medical alternatives that require further exploration to understand their application as viable therapeutics for neurotrophic keratitis. [21]
Despite these measures, corneal neurotization exists as a potentially long term solution with proven increases in corneal sensitivity. It exists as an option that may preclude the financial and logistical burden of many of the medically available or theoretical treatment options for neurotrophic corneas.
Preoperative Evaluation
The preoperative evaluation should first evaluate the general state of ocular and periorbital health and root cause of corneal anesthesia before more in-depth quantitative preoperative evaluation occurs. The patient’s visual acuity, meibomian gland function, lacrimal gland function and corneal status should be assessed.
Then, patients should be identified for having active infectious, inflammatory, neoplastic, or immunocompromising conditions which may be contributing to the corneal anesthesia. Patients with these ongoing processes, and those with active radiotherapy or chemotherapy may not qualify as candidates for immediate corneal neurotization. Due to the progressive nature of neurotrophic keratitis, addressing the underlying cause of corneal denervation should be considered prior to progressing with surgery.
The preoperative evaluation should then focus on the viability of the donor nerves, as well as the corneal sensitivity of the ophthalmic division of cranial nerve five (trigeminal). The nerves responsible for initiating blink reflexes, such as the nasociliary branch and the long ciliary nerve branches, can be tested via cotton swabs or esthesiometry. Although corneal sensitivity can be determined using cotton swabs, a more precise quantitative approach for testing corneal sensitivity severity is via esthesiometry 86.[22]
Esthesiometry
Quantitative corneal sensation may be assessed with Cochet-Bonnet esthesiometer, which has a maximum length of 60 mm (most sensitive). The Cochet-Bonnet esthesiometer is a handheld device which tests corneal sensitivity using a 0.12 mm caliber nylon filament [10]. Testing begins with the nylon filament fully extended to 60 mm, the expected normal sensitivity threshold, and placed perpendicularly on the cornea. If no response is elicited, the nylon filament is shortened by increments of 10mm until a response is elicited. Shortening the filament increases mechanical pressure placed on the eye, disclosing sensory damage severity. Esthesiometry readings can be generalized into 4 groups: absent, low, moderate, and high sensitivity. Using Cochet-Bonnet, below 20mm is low sensitivity, 30-40mm is moderate, and above 50mm is high sensitivity. [10]
Confocal Microscopy
In-vivo confocal microscopy is an option to obtain both preoperative and postoperative objective measurements of the cornea. First cited for application in corneal neurotization by Fung et al., in-vivo confocal microscopy is a useful, noninvasive tool that produces histology-like images. These high resolution images have previously been obtained for use in assessing the cornea after keratoplasty, laser refractive surgery, and in neurotrophic corneas after autologous plasma or murine nerve growth factor, and may now be used in corneal neurotization. [23] Preoperatively, it may be used to assess the severity of damage to the corneal epithelium, the stroma, endothelium, and the sub-basal neural plexus. These images can serve as a helpful baseline to compare against after surgical intervention.
Donor Nerve Selection
The nerve selection between the supratrochlear, supraorbital, or infraorbital must be determined. To dictate which donor nerve will participate, ipsilateral or contralateral, is decided by sensation of donor nerve, size of nerve, physicians preference, and distance between nerve terminal and cornea .[6] The supraorbital and infraorbital ipsilaterally are preferred nerves as they have a high axon count (6000 myelinated axons) and are closest to the cornea. [6] Supratrochlear nerve remains a viable option, but has a lower axon count (2500 myelinated axons) and the anatomical nerve pathway is not as consistent as the infraorbital option. By testing sensation of the supratrochlear nerve (innervates the medial forehead, upper eyelid and the medial portion of the nose), supraorbital nerve (innervates skin of the lateral forehead, anterior scalp, upper eyelid, and conjunctiva of the upper eyelid), and infraorbital nerve (innervates cheek, upper lip, lateral aspect of the nose, and upper gingiva/teeth) a donor nerve can be selected based on functional sensitivity. If ipsilateral nerves have decreased function, further testing for the great auricular nerve should be conducted.[6] Once ipsilateral nerve options have been exhausted, testing of contralateral nerves would be appropriate.
Additionally, due to individual variation in anatomy, it is also appropriate to once again assess best fit between supraorbital and supratrochlear nerves intraoperatively. Given that the preoperative sensibility testing is intact equally between the two nerves, caliber of donor pedicles and allograft may be used as an assessment parameter of best fit.
Special Considerations
It is recommended to give special consideration to patients with a history of zoster-induced keratopathy during nerve donor selection. Per the literature, it is not well-elucidated if the use of an unaffected contralateral nerve can spread the virus.
Further consideration must also be taken for patients with extensive or prior ocular surgery. Past glaucoma, corneal, or retinal surgery may have produced corneal or conjunctival scarring which may limit access and preclude typical surgical approaches. The placement of previous hardware may also limit access to areas which need graft placement.
Severity Grading
Mackie Stages
There are varying degrees of damage to the cornea based on the severity of neurotrophic keratitis. Different classification systems have arisen over time. The first classification system created in 1995, the Mackie stages, grade severity from mildest (stage 1) to most severe (stage 3) as follows: [9][3]
- Stage 1 (Mild): Characterized by epithelial surface disruptions such as punctate keratopathy. There is often epithelial hyperplasia, reduced tear breakup time, and superficial neovascularization. There may also be conjunctival staining with Rose Bengal in this stage, which indicates involvement of the epithelium of the conjunctiva.
- Stage 2 (Moderate): The superior portion of the cornea commonly presents with persistent defects of the epithelium that tend to be circular or oval in shape. The edges of the epithelial defect may be smooth or rolled, and surrounding edema may exacerbate the defect, leading to spontaneous detachment. Descemet’s folds and anterior chamber inflammation with hypopion may also be present.
- Stage 3 (Severe): Characterized by frank corneal ulcers that lead to corneal perforations or corneal stromal melt.
Combined System of Grading
With the incorporation of more technology, a second classification system was described by DiZazzo et al., in 2018. This newer system of grading neurotrophic keratitis accounts for not only the clinical and anesthesiometric parameters of corneal sensitivity, but also includes the confocal microscopic characteristics of the corneal layers and Anterior Segment Optical Coherence Tomography (AS-OCT) quantitative analysis of thinning of the stroma. [7]
| Stage | In Vivo Confocal Microscopy | Mean Nerve fiber density | Total number of nerves per mm2 | Anterior Segment OCT findings |
| 1 (mild) |
|
10.06 ± 0.51 | 1.8 ± 0.79 per mm2 |
|
| 2 (moderate) |
|
3.11 ± 0.39
µm/mm2 |
0.7 ± 0.8 per mm2 |
|
| 3 (severe) |
|
0.33 ± 0.37 µm/mm2 . | 1 ± 0.4 per mm2 |
|
Relevant anatomy
Direct Neurotization
Direct neurotization most frequently utilizes the supraorbital and supratrochlear branches of the frontal nerve. The two nerves exit the orbit above the level of the superior orbital rim. The supraorbital nerve exits specifically at the supraorbital notch while the supratrochlear nerve exits 21 mm from the midline between the supraorbital notch and the pulley of the superior oblique. These nerves may be difficult to mobilize as they generally exit through the orbital rim foramina. [24]
The sensory function of the two nerves overlap over the medial forehead, so harvesting one for a corneal neurotization surgery should theoretically leave adequate residual sensation. Generally, thicker and denser nerve fibers are thought to have greater potential for neurotization, so the supraorbital nerve is preferentially harvested over the supratrochlear nerve. [25][26][27]
Indirect Neurotization
Indirect neurotization most frequently utilizes the sural nerve due to its accessibility and is a historically low risk donor graft site in other neurotization procedures. [11] [25][27]The sural nerve is connected from the intact sensory donor nerve to the anesthetic eye. The sural nerve provides sensation to the calf region of the leg and consists of branches from the tibial and common fibular nerve.
The great auricular nerve is a cutaneous superficial nerve which arises from the cervical plexus and originates from the C2-C3 levels. It is responsible for sensory function to the skin over the parotid, mastoid processes, and surfaces of the ears. The great auricular nerve has been used in corneal neurotization surgeries and one advantage is its proximity to the recipient site as well as the need to only prepare one surgical field.[25]
Surgical Technique
As is the concern of every surgeon, success of surgical approach and technique always remains a primary consideration. However, among the various surgical approaches, none have demonstrated superiority over others to date.[28] Direct neurotization offers less chances of scarring but limitations exist in nerve length and need for extensive dissection.
Indirect neurotization offers a less extensive procedure but may theoretically have decreased nerve fibers due to perineural scarring. Selection of the proper approach relies on patient anatomy, site of damage, patient preference, surgeon expertise and comfort. Indirect neurotizations utilize coaptations via an end-to-end, or end-to-side approach, although studies have not definitely demonstrated superiority of one technique over the other. However, considering surgical ease, attaching the graft end to the donor nerve side may be technically less demanding.
There are various surgical approaches and, to date, none have been demonstrated to be superior over others. Direct neurotization offers less chance of scarring but limited in nerve length and need for extensive dissection. Indirect neurotization offers a less extensive procedure but may theoretically have decreased nerve fibers due to perineural scarring[25]. Selection of the proper approach relies on patient anatomy, site of damage, patient preference, surgeon expertise and comfort. Surgeons connect the nerves end-to-end, or end-to-side, although studies have not definitely demonstrated superiority of one technique over the other. Attaching the graft end to the donor nerve side may be technically easier.[6]
Direct Neurotization
While ipsilateral grafts may shorten the operative time and extent of dissection compared to contralateral grafts, this option is only open if solely the ipsilateral long ciliary nerves are damaged. Otherwise, the chance of trigeminal nerve compromise will preclude the usefulness of its distal branches.
Contralateral Supraorbital/Supratrochlear Nerve Direct neurotization
Overview, Advantages, and Disadvantages
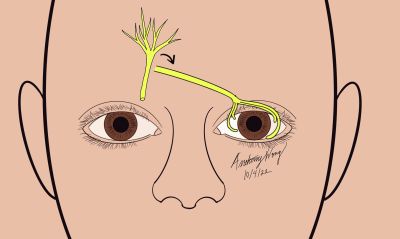
First Described by Terzis et al,[10] the direct neurotization involves using the patient’s contralateral supraorbital or supratrochlear nerve to reinnervate the anesthetic cornea. Once accessed, the nerve is tunneled and placed at the limbus of the affected eye. The disadvantage of this traditional approach is that it involves creating a large bicoronal incision and may lead to subgaleal hematoma, alopecia, and prolonged surgical time.
Surgical Approach (Adapted from Terzis et al) [10][24][25] [27]
- Prepare and drape the patient in a standard sterile fashion
- Create a bicoronal incision across the scalp and reflect it inferiorly
- Identify and dissect the contralateral supraorbital/supratrochlear nerve through the bicoronal incision and flap proximal to supraorbital margin
- Tunnel the supraorbital/supratrochlear nerve branches subcutaneously, crossing the nasal bridge to the neurotrophic eye
- Create a small supratarsal incision and thread the nerve branches out toward the incision
- Using a small hemostat, carefully retrieve the distal nerve branches and Tunnel the nerve branches through the blepharotomy into the superior conjunctival fornix
- Create space for nerve fascicles by creating an Incision through the superonasal bulbar conjunctiva and sub tenon’s space 7 mm from the limbus
- Perform Blunt Dissection around the limbus (360 degrees) to create tunnels in the potential space between the the sclera and Tenon’s space at their junction at the limbus
- Dissect nerve epineurium to expose fascicles
- Distribute fascicles to the perilimbal area, and secure the nerve fascicles to the limbus with with 10-0 nylon on a spatulated needle
- Repair conjunctival incisions with buried sutures
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Ipsilateral supraorbital/supratrochlear Nerve Direct neurotization
Overview, Advantages, and Disadvantages
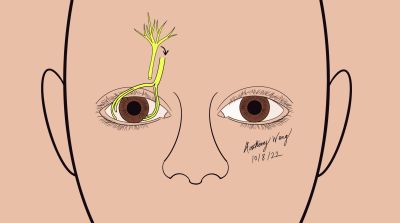
An ipsilateral approach to neurotization was later completed by Jacinto et al.[24] Instead of using a bicoronal incision, this technique involved a hemicoronal incision with subsequent tunneling and transfer of the supraorbital or supratrochlear nerve inferiorly to the anesthetic cornea. This technique bypasses the need for a more extensive bicoronal incision; however, the hemicoronal incision is still a relatively invasive approach.
Surgical Approach (Adapted from Jacinto 18)[10][25][27][24][29]
- Prepare and drape the patient in a standard sterile fashion
- Create a hemicoronal incision just behind the hairline hairline starting from the midline and extending to the auricular helix
- Isolate and Identify the ipsilateral supraorbital/supratrochlear nerves
- Due to individual anatomic variability, perform intraoperative assessment of best fit to select between supratrochlear or supraorbital donor nerves
- Perform blunt dissection in the subgaleal plane, laterally over the deep temporal fascia and inferiorly until 2 cm above the superior orbital rim
- Continue dissection subperiosteally until the superior orbital rim and dissect the deep branch of the ipsilateral supraorbital nerve, harvesting 3 branches which are then cut 6 cm from the supraorbital notch
- Create an upper eyelid incision and tunnel the nerve fibers through the supratarsal incision along the upper lid crease
- Tunnel the nerve further through the blepharotomy incision into the superior conjunctival fornix
- Dissect the bulbar conjunctiva perilimbally and under Tenon’s capsule to create space for the nerve branches
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Distribute fascicles to the perilimbal area, and secure the nerve fascicles to the limbus with with 10-0 nylon on a spatulated needle
- Repair conjunctival incisions with buried sutures
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Minimally Invasive Transblepharoplasty Ipsilateral Supraorbital/Supratrochlear Nerve Approach
Overview, Advantages, and Disadvantages
This minimally invasive approach employed by Phelps and also by Wisely utilizes an upper eyelid crease incision and/or smaller forehead incisions to gain access to the supraorbital/supratrochlear nerve. This technique’s advantage lies in avoidance of the traditional large hemicoronal or bicoronal incisions previously needed to identify and mobilize the donor nerves. Additionally, this method may be preferred due to its more aesthetically pleasing result.
Surgical Approach (Adapted from Phelps, Wisely [17])
- Prepare and drape the patient in a standard sterile fashion
- Create an upper eyelid (typical blepharoplasty) incision and dissect through the orbicularis oculi muscle, orbital septum, and down to the orbital rim
- Continue dissection down to the supraorbital rim and identify the supraorbital/supratrochlear nerve
- Create a medial brow incision and perform blunt dissection to separate the nerve from surrounding tissue until the nerve is exposed in the dorsal subcutaneous plane
- Create a mid-forehead incision 3 mm above the brow and isolate and identify the distal branch of the supraorbital/supratrochlear nerve
- Due to individual anatomic variability, perform intraoperative assessment of best fit to select between supratrochlear or supraorbital donor nerves
- Pass the distal ends of the nerve branches down to the supraorbital rim (to facilitate movement of the nerves, a neural hitch technique may be used by passing 4-0 silk though the ends of the nerves)
- Tunnel the nerve further through the blepharotomy incision into the superomedial conjunctival fornix
- Incise and dissect the bulbar conjunctiva around the corneoscleral limbus to create space for the nerve branches
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Distribute fascicles to the perilimbal area (longest branches at the 9 O’clock positions, shorter branches to the 10 and 12 O'clock positions) and trim the excess portions of the nerve fascicles to portions which are viable
- Secure the nerve fascicles to the limbus with with 10-0 nylon on a spatulated needle
- Repair conjunctival incisions with buried sutures
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Ipsilateral Infraorbital Nerve Direct Neurotization
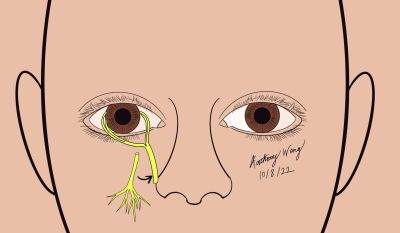
Overview, Advantages, and Disadvantages
Corneal Neurotization with the ipsilateral infraorbital nerve as the donor nerve is another alternative approach which may be useful in the event that the supraorbital or supratrochlear nerves are not viable. This technique has shortcomings in the need for extensive and delicate dissection as well as a limited graft length.
Surgical Approach (Adapted from Leyngold [30])
- Prepare and drape the patient in a standard sterile fashion
- Make an incision in the inferior conjunctival fornix and perform blunt dissection down to the infraorbital rim
- Expose the orbital floor and the orbital groove
- Using an osteotome, gently unroof the infraorbital canal
- With Takahashi Rongeurs, remove the bone over the canal and elevate the nerve over the canal
- Dissect the inferior bulbar conjunctiva at the limbus and dissect into the sub-Tenon’s plane to create space for the nerve fasicles
- With the nerve freed, transfer the nerve via the tunnel to its appropriate perilimbal position
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Distribute fascicles to the perilimbal area and trim the excess portions of the nerve graft to portions which are viable
- Secure the nerve fascicles to the limbus with with 10-0 nylon on a spatulated needle Note: fibrin glue may also be used to secure fascicles to the limbus as demonstrated by Leyngold
- Repair conjunctival incisions with buried sutures, ensuring fascicles are covered completely
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Endoscopic Approach with supraorbital/supratrochlear nerve Direct neurotization
Overview, Advantages, and Disadvantages
In the literature, an endoscopic approach to corneal neurotization has been performed. Its advantages lie in the avoidance of a large hemicoronal or bicoronal incision that was first described in early corneal neurotization surgeries. It also reduces the inherent risks involved with large incision surgeries which include large and visible scarring, subgaleal hematoma, alopecia, and prolonged surgical time. The drawback is the need for additional equipment and proficiency with its use.
Surgical Approach (Adapted from Leyngold et al.,[31])
- Prepare and drape the patient in a standard sterile fashion
- Administer 1% lidocaine with 1:100,000 epinephrine (diluted 1:9 with normal saline) to the central forehead region
- Create an upper eyelid incision on the side contralateral to the anesthetic eye. This will serve as the donor side
- Dissect deep toward the superior orbital rim until the supraorbital nerve is identified
- Dissect superiorly in the subgaleal plane until it is possible to isolate a 1 cm portion of the supraorbital nerve branch
- Create two 1 cm vertical incisions approximately 5 mm posterior to the trichion, with the first incision at the midline, and the second incision lining up with the contralateral medial limbus
- Create a plane within the subperiosteal space using an endoscopic elevator and dissect inferiorly, stopping just superiorly to the previously isolated supraorbital nerve branch
- Open the periosteum to visualize the supraorbital nerve, and endoscopically dissect the supraorbital nerve cephalad from both the eyelid and scalp incisions
- Isolate and divide two branches of the supraorbital nerve (measuring 7 cm superior to the supraorbital notch)
- Create an upper eyelid incision on the recipient side (anesthetic eye)
- Tunnel the nerves and pass them through the subgaleal plane, crossing the nasal bridge and out through the blepharotomy sites
- Create a superior medial conjunctival fornix incision and pass the nerves through to arrange them on the globe
- Incise and dissect the superior bulbar conjunctiva around the corneoscleral limbus to create space for the nerve branches
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Distribute fascicles to the perilimbal area, and suture fixate with 10-0 nylon and a spatulated needle
- Repair conjunctival incisions with buried sutures, ensuring fascicles are covered completely
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Indirect Neurotization
Indirect neurotization is a useful technique to achieve neurotization. In these cases an interpositional graft is connected to the donor nerve to the anesthetic cornea. Either an end to end neurorrhaphy or end to side coaptation may be used to attach the nerve graft to the donor nerve.
Sural nerve graft to ipsilateral or contralateral supraorbital/supratrochlear nerve Indirect Neurotization
Overview, Advantages, and Disadvantages
This indirect approach uses a sural nerve as an interpositional graft between the donor nerve and the affected anesthetic eye. It also employs a superior eyelid crease incision to gain access to the donor nerve. An advantage to this approach is the ability to harvest a graft of substantial length. The disadvantages lie in the need for a second surgical site and the need for the nerve to heal through two junctions.
Surgical Approach (Adapted from Elbaz [32], Bains [33])
- Prepare and drape the patient in a standard sterile fashion, Note: there will be two: one for the lower extremity and one for the face
- Create two or three short transverse posterior leg incisions to access the sural nerve (one distal to popliteal fossa, another inferiorly around the mid to low calf region)
- Once located proximally, use a wire-loop nerve stripper and place it around the nerve. Advance the device distally until the desired length of nerve graft is achieved (10-15cm) and divide the nerve at its distal end
- Create a small transverse upper eyelid incision and dissect superiorly deep to the orbicularis oculi muscle to gain access to the donor supratrochlear/supraorbital nerves
- Create an epineural window and perform end-to-side coaptation of the sural nerve graft
- Note: End-to-side coaptation may be selected to preserve forehead sensation; however, if the nerves are of limited caliber, an end to end coaptation may be employed
- **For contralateral cases: Create an incision in the contralateral upper eyelid and tunnel subcutaneously contralaterally across the nasal bridge
- Create an incision in the superior medial conjunctival fornix for the supratrochlear nerve to pass inferiorly
- Incise and dissect the bulbar conjunctiva around the corneoscleral limbus to create space for the nerve branches
- Transfer the sural nerve graft through the tunnel, down through the superior medical conjunctival fornix incision and arrange it around the limbus
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Distribute fascicles to the perilimbal area, and suture fixate with 10-0 nylon and a spatulated needle
- Repair conjunctival incisions with buried sutures, ensuring fascicles are covered completely
- Perform an end to side coaptation at the proximal end of the nerve with 10-0 nylon and/or fibrin glue after making an epineurial window (note: an end to end coaptation may be used if the nerve is small)
- Close the skin superior to the donor site
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Greater Auricular Nerve Interpositional Graft to Contralateral Supratrochlear Nerve Indirect Neurotization
Overview, Advantages, and Disadvantages
This approach utilizes the greater auricular nerve as an interpositional graft between the donor supratrochlear nerve and the anesthetic eye. Advantages are the avoidance of a second surgical field and use of a donor nerve that may be useful if other donor sites are not viable. The drawback is the need for a neck incision and the potential need for a second surgical team to aid in harvesting the greater auricular nerve. The nerve graft will also need to heal through two surgical junctions.
Surgical Approach (Adapted from Benkhatar [34])
- Prepare and drape the patient in a standard sterile fashion
- Perform a 3 cm incision into the neck into the middle portion of the greater auricular nerve (exit point of the nerve lies approximately 6.5 cm from the mastoid and 1 cm from the external jugular vein)
- Remove the greater auricular nerve trunk and a portion of the posterior branch below the mastoid process
- Create a 1 cm medial brow incision and dissect deep to identify and isolate the supratrochlear nerve
- Dissect until around 1 cm of supratrochlear nerve length is released superiorly
- Create a 1 cm skin incision in the infrabrow region on the side of the affected eye
- Tunnel inferiorly and subcutaneously above the glabella and extend toward the skin incision previously made
- Pass the greater auricular nerve graft from the contralateral supratrochlear nerve through the tunnel and through an incision in the superior conjunctival fornix to reach the bulbar conjunctiva of the anesthetic eye
- Perform an end to end neurorrhaphy between the greater auricular nerve graft and the supratrochlear nerve with 10-0 Nylon epineural sutures and match the ends of the nerves appropriately
- Incise and dissect the bulbar conjunctiva around the corneoscleral limbus to create space for the nerve branches
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Distribute fascicles to the perilimbal area, and suture fixate with 10-0 nylon and a spatulated needle
- Repair conjunctival incisions with buried sutures, ensuring fascicles are covered completely
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Sural Nerve Interpositional Graft to Ipsilateral Greater Auricular Nerve Indirect Neurotization
Overview, Advantages, and Disadvantages
In this procedure, the typical supraorbital and supratrochlear nerves are spared and instead, the greater auricular nerve is selected as the donor site. The sural nerve is utilized as a graft to connect the greater auricular nerve to the anesthetic cornea on the ipsilateral side. Advantages lie in harvesting a graft of substantial length and caliber. Disadvantages include need for a second surgical site and a more involved process of harvesting the graft.
Procedural Steps Adapted from (Jowett and Pineda [35] )
- Prepare and drape the patient in a standard sterile fashion
- Perform a 3 cm incision into the neck to access the terminal branches of the greater auricular nerve on the ipsilateral side of the affected eye (exit point of the nerve lies approximately 6.5 cm from the mastoid and 1 cm from the external jugular vein)
- Harvest the sural nerve graft via endoscopic approach and select the medial cutaneous portion for the interpositional graft
- Note technique from Elbaz and Bains may be used if not endoscopically:
- Create two or three short transverse posterior leg incisions to access the sural nerve (one distal to popliteal fossa, another inferiorly around the mid to low calf region)
- Once located proximally, use a wire-loop nerve stripper and place it around the nerve. Advance the device distally until the desired length of nerve graft is achieved (10-15cm) and divide the nerve at its distal end
- Note technique from Elbaz and Bains may be used if not endoscopically:
- Perform an inferior peritomy that is 7mm posterior to the limbus (U shaped and inferior fornix-based)
- Pass a Wright fascia needle from the subconjunctival plane inferior and posterior to the sub superficial musculoaponeurotic system plane of the cheek and out through the infra-auricular incision
- With the nerve end threaded with the needle, carefully pull the nerve through the previously created space so that it is arranged between the greater auricular nerve and the affected anesthetic eye
- Incise and dissect the bulbar conjunctiva around the corneoscleral limbus to create space for the nerve branches
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Trim the excess portions of the nerve fascicles to portions which are viable
- Distribute fascicles to the perilimbal area, and suture fixate with 10-0 nylon and/or fibrin glue
- Repair the inferior conjunctival flap with fibrin glue, ensuring fascicles are covered completely
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
- Trim the sural nerve graft to an appropriate length and coapt to branches of the greater auricular nerve using 10-0 nylon sutures
- Close the neck incision
Ipsilateral Infraorbital Nerve Indirect Neurotization
Overview, Advantages, and Disadvantages
The indirect variation of corneal neurotization with the ipsilateral infraorbital nerve was described by Leyngold. This approach is useful in the event that the supraorbital or supratrochlear nerves are not viable. As with the direct approach, the shortcomings lie in the need for extensive and delicate dissection as well as a limited graft length. However, its end to side coaptation typically results in faster sensory recovery than end to end coaptation. [30]
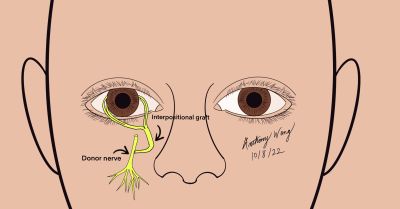
Surgical Approach (Adapted from Leyngold [30])
- Prepare and drape the patient in a standard sterile fashion
- Make an incision in the inferior conjunctival fornix and perform blunt dissection down to the infraorbital rim
- Expose the orbital floor and the orbital groove
- Using an osteotome, gently unroof the infraorbital canal
- With Takahashi Rongeurs, remove the bone over the canal and elevate the nerve over the canal
- Perform an end to side coaptation between the nerve allograft (or other harvested nerve) and the infraorbital nerve
- An epineureal window is made on the anterior surface of the infraorbital nerve
- Perform an end to side neurorraphy with 9-0 nylon suture
- Dissect the inferior bulbar conjunctiva at the limbus and dissect into the sub-Tenon’s plane to create space for the nerve fasicles
- Transfer the nerve via the tunnel to its appropriate perilimbal position
- Dissect nerve epineurium of the ocular end of the nerve graft to expose fascicles
- Distribute fascicles to the perilimbal area and trim the excess portions of the nerve graft to portions which are viable
- Secure the nerve fascicles to the limbus with with 10-0 nylon on a spatulated needle Note: fibrin glue may also be used to secure fascicles to the limbus as demonstrated by Leyngold
- Repair conjunctival incisions with buried sutures, ensuring fascicles are covered completely
- Temporal tarsorrhaphy may be considered to facilitate healing and protection of the cornea in the early postoperative period
Minimally Invasive/ Acellular Nerve Allograft for Indirect Neurotizations
As developments have occurred in the realm of corneal neurotization, Leyngold et al.[30], discussed a novel approach to bypass the typical use of host-derived interpositional grafts and direct nerve transfers. This technique employs acellular nerve allografts (Avance Nerve Graft, AxoGen) for indirect neurotization with good results.
This approach is not a standalone technique, but can instead be used in the case of all indirect neurotizations with an interpositional graft. To employ this surgical approach, one would first gain access to the donor nerve. Instead of using an interpositional nerve graft, processed acellular nerve allograft (Avance Nerve Graft) measuring 70mm × 1–3mm can be coapted to the donor nerve. To improve protection for an end to end neurorrhaphy, an amniotic membrane graft or nerve connector wrap may be used.
Advantages to this approach lie in its minimally invasive approach and avoids the need to harvest an autologous graft from the patient. However, the disadvantages are cost of the nerve allograft and theoretical inferiority of the processed acellular autograft to an autologous graft.
Surgical Approach Comparison
| Approach | Advantages | Disadvantages |
| Direct Approaches | ||
| Contralateral supraorbital/supratrochlear Nerve Direct neurotization (Bicoronal Incision) | -Bypasses use of interpositional graft and second surgical site | -Large bicoronal incision with risk of significant scarring
-Risk of alopecia -Risk of Subgaleal hematoma -Extensive operative time |
| Ipsilateral supraorbital/supratrochlear Nerve Direct neurotization (Hemicoronal Incision) | -Bypasses use of interpositional graft and second surgical site
-Hemicoronal incision is less invasive than bicoronal approach |
-Large hemicoronal incision still presents with risk of significant scarring
-Risk of alopecia -Risk of Subgaleal hematoma -Extensive operative time |
| Minimally Invasive Transblepharoplasty Ipsilateral Supraorbital/Supratrochlear Nerve Approach | -Bypasses use of interpositional graft and second surgical site
- Eyelid incision is aesthetically more pleasing -No need for special equipment -Avoids sequelae of traditional large incision |
-Limited in extent to length of nerve harvested (maximum 3-4 cm) without secondary incision |
| Ipsilateral Infraorbital Nerve Direct Neurotization | -Bypasses use of interpositional graft and second surgical site
-Avoids interpositional graft and second surgical site |
-Limited in extent to length of nerve harvested (maximum 3-4 cm)
- Approach requires delicate and involved dissection - Greater sequelae of nerve morbidity over previously anatomically supplied region (numbness of upper lip, teeth, cheek) |
| Endoscopic Approach with supraorbital/supratrochlear nerve Direct neurotization | -Less invasive approach avoids large bicoronal or hemicoronal incisions
-Bypasses use of interpositional graft and second surgical site - Eyelid incision is aesthetically more pleasing -Can be used for ipsilateral or contralateral approaches |
-Risk of alopecia
-Proficiency with and Availability of endoscopic equipment is required |
| Indirect Approaches | ||
| Sural nerve graft to ipsilateral or contralateral supraorbital/supratrochlear nerve Indirect Neurotization | - Eyelid incision is aesthetically more pleasing
- Harvested nerve can be of greater length |
- A second incision and surgical field is needed for the sural nerve graft harvest
-A second surgical team may be needed for the graft harvest -Extensive operative time -Nerve graft must heal through two surgical junctions |
| Greater Auricular Nerve Interpositional Graft to Contralateral Supratrochlear Nerve Indirect Neurotization | - Nerve donor site and interpositional nerve graft harvest site can be in one surgical field | - A second incision in the neck is needed for the greater auricular nerve graft harvest
-A second surgical team may be needed for the graft harvest -Extensive operative time -Nerve graft must heal through two surgical junctions |
| Sural Nerve Interpositional Graft to Ipsilateral Greater Auricular Nerve Indirect Neurotization | - Harvested nerve can be of greater length | - Neck incision is required to access greater auricular nerve
-The nerve graft must travel over a long distance - A second incision and surgical field is needed for the sural nerve graft harvest -A second surgical team may be needed for the graft harvest -Extensive operative time -Nerve graft must heal through two surgical junctions |
| Ipsilateral Infraorbital Nerve Indirect Neurotization | Avoids sensory loss in the supraorbital and supratrochlear area | -A second surgical team may be needed for the graft harvest
-Longer operative time -Approach requires delicate and involved dissection - Greater sequelae of nerve morbidity over previously anatomically supplied region (numbness of upper lip, teeth, cheek) -Nerve graft must heal through two surgical junctions |
| Minimally Invasive/ Acellular acellular nerve interpositional allograft | - More aesthetically pleasing incision
-Operative time is decreased -Bypasses need to harvest an autograft -Avoids need for second surgical field and incision |
-Difficult acquisition of acellular nerve allograft due to cost
-Theoretical inferiority of allograft to autologous graft |
Postoperative management
Patients may be patched for a short time postoperatively for approximately one to two days and discharged with non-opioid pain medications. Prophylactic antibiotic drops should be prescribed two to four times daily for one to two weeks, along with lubricating drops. Some case reports in the literature utilized steroid drops. An antibiotic ointment (erythromycin) should also be prescribed twice daily for two weeks applied over the skin incisions.
The patient should also be instructed to avoid heavy lifting or strenuous physical activity for 2 weeks after surgery. In cases of patients where a protective tarsorrhaphy was placed, it should be released at one week’s time or sooner depending on individual rate of healing. Remaining skin sutures are removed at the appropriate followup appointments, and corneal sensation is measured with esthesiometry or less quantitatively with the cotton wisp test.
Follow Up Evaluation
Postoperative follow up schedule may be planned as follows: 1 day, 1 week, and 1, 3, 6, 9, 12, and 24 months after surgery. Given the long evolution of improvement typically associated with corneal neurotization, long term follow up is advisable.
At follow up, subjective and objective measures of corneal sensibility should be assessed. Patients should be evaluated for new or recurrent punctate epithelial erosions, epithelial defects, ulceration, neovascularization, scarring, corneal melt and perforation, and/or denervation.
Esthesiometric testing should be completed to monitor objective improvement in corneal sensitivity. Confocal microscopy can also be used to give objective measures of corneal reinnervation after corneal neurotization. If baseline images were taken, in vivo confocal microscopic images can help visually illustrate trophic increases in nerve sprouts in addition to improvements in other layers of the cornea.
Complications
As such with all surgeries, corneal neurotization is not exempt from complications and unintended outcomes.
- Infection, surgical failure, need for reoperation, hemorrhage
- Common in all surgical procedures, these complications exist, but can be mitigated through the use of antibiotics, proper technique, and close follow up, vasoconstrictive agents, and cautery
- Significant Scarring at the incision site [6]
- In Direct neurotization cases with large bicoronal or hemicoronal incisions, the scarring may be unsightly and noticeable. Indirect neurotization cases which involve harvesting a graft from other locations (sural nerve, greater auricular nerve) may also leave patients with a large scar
- Donor site numbness, pain, and discomfort
- Numbness in the anatomically supplied region of the donor nerve is a common outcome after corneal neurotization, especially if a nerve is harvested for use as a graft. Pain and itching have been noted in the donor site. Discomfort is also a reported side effect, but usually dissipates after a few months[21]
- Alopecia
- With incisions made with hemicoronal, bicoronal, or posterior to the hairline, alopecia is an unintended consequence. There is a reduced risk of this complications with endoscopic or minimally invasive approaches. [6]
- Subgaleal Hematoma Formation
- Reports of postoperative subgaleal hematoma exist, especially with larger, more invasive techniques involving a bicoronal or hemicoronal incision and dissection. As with alopecia, there is a reduced risk with an endoscopic or minimally invasive approach. [6]
- Neuroma Formation
- Neuroma formation has been listed as an adverse effect of corneal neurotization. There is a particular chance of this complication at the site of neurorrhaphy. Nerve wraps or amniotic membrane wraps may be used to protect the site of coaptation and prevent axonal escape and formation of a neuroma.[6]
- Reactive bony overgrowth at the site of dissection
- Neuropathic pain
- Although relatively uncommon, the risk of neuropathic pain exists and has been reported in the literature[6]
- Delayed corneal reinnervation
- Indirect approaches to corneal neurotization face potential for slower reinnervation due to the need for the nerves to grow through two surgical junctions
- Failure of Neurorrhaphy
- Especially in indirect cases, the site of neurorrhaphy may fail and axonal escape may occur. This will result in surgical failure and potential neuroma formation
- Hyperesthesia
- Hyperesthesia, although relatively uncommon, exists as a potential complication of corneal neurotization in the literature. [6]
- Referred Pain
- Referred pain and tactile sensation, although not necessarily a complication, may occur in the postoperative period. Typically, these effects resolve within 3-6 months, but one case has reported a 10 year period of ocular referred discomfort [6]
- Abnormal Sensations
- Although not explicitly clear, there have been reports of patients undergoing greater auricular nerve interpositional grafts that have lasting abnormal earlobe sensations after surgery [35]
Outcome and Prognosis
Overall, corneal neurotization has been met with general success and good prognosis. Although the degree to which corneal sensibility is restored varies in the literature, the aggregate results show significant improvement from pre-operative states. There are also increasing reports of success in treating children with neurotrophic keratopathy with corneal neurotization.
Restoration of corneal sensation may occur as early as one-week postoperatively, which may paradoxically manifest as worsening ocular pain and discomfort. The maximal rate of nerve growth from the limbus into the cornea is 1mm/day, but typically occurs slower. [33] During the healing phase an interesting phenomenon may be observed where the patient can feel the donor nerve concomitant with corneal stimulation, and vice versa. Over time, patients usually experience an increase in subjective corneal sensation followed by objective improvement and consequent reduction in corneal vascularization. [25][24]
Complete neurotization of the basal and central cornea may be seen from 6 months to 2 years post-operatively, and long term success is affected by the patient's age and comorbidities. Patients usually experience improvements in visual acuity, corneal sensation, and reduction in symptoms in the months after surgery and will maintain these improvements over years. [10][36][24][31][32][30][37][38]Patients may even expect maintenance of corneal sensation even up to 20 years post-operation.
In the seminal report by Terzis et al, the subjective time to improvement in sensitivity was reported between 6 months and 1 year, and time to objective sensibility was 2.80 +/- 2.17 years. [10] The average time to denervation was 7 +/- 8.56 years. The outcome of corneal sensation measured by esthesiometry was >50 (high) in 1 patient, moderate (>20-<50 mm) in 2 patients, low (≤20 mm) in 2 patients, and not available in 1 patient after an average of 16 +/- 2.4 years. In a recent meta analysis, improvements on the Mackie grading scale showed a statistically significant improvement of corneas after direct and indirect corneal neurotization. The mean improvement was from 2.46 +/- 0.77 to 0.86 +/- 0.79. [6]
It is important to note that improvement in corneal sensitivity had no correlation to the number of nerve bundles used throughout the procedure. [10] It is thought that the physiology of reinnervation likely occurs from a combination of direct sprouting from proximal nerve endings (after Wallerian degeneration of the distal) and release of neurotrophic factors.
Additional Resources
- Domeshek LF, Hunter DA, Santosa K, Couch SM, Ali A, Borschel GH, Zuker RM, Snyder-Warwick AK. Anatomic characteristics of supraorbital and supratrochlear nerves relevant to their use in corneal neurotization. Eye (Lond). 2019 Mar;33(3):398-403.
- Koaik M, Baig K. Corneal neurotization. Curr Opin Ophthalmol. 2019 Jul;30(4):292-298.
- Malhotra R, Elalfy MS, Kannan R, Nduka C, Hamada S. Update on corneal neurotisation. Br J Ophthalmol. 2019 Jan;103(1):26-35.
- Leyngold IM, Kossler AL, Yen MT. Techniques in Corneal Neurotization. Quality Medical Publishing, Incorporated, 2020
References
- ↑ Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003 Nov;17(8):989-95. doi: 10.1038/sj.eye.6700616. PMID: 14631406.
- ↑ ZANDER E, WEDDELL G. Observations on the innervation of the cornea. J Anat. 1951 Jan;85(1):68-99. PMID: 14814019; PMCID: PMC1273614.
- ↑ Jump up to: 3.0 3.1 Mastropasqua L, Nubile M, Lanzini M, Calienno R, Dua HS. In vivo microscopic and optical coherence tomography classification of neurotrophic keratopathy. J Cell Physiol. 2019 May;234(5):6108-6115. doi: 10.1002/jcp.27345. Epub 2018 Sep 21. PMID: 30240004.
- ↑ Jump up to: 4.0 4.1 Yang AY, Chow J, Liu J. Corneal Innervation and Sensation: The Eye and Beyond. Yale J Biol Med. 2018 Mar 28;91(1):13-21. PMID: 29599653; PMCID: PMC5872636.
- ↑ Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981 Dec;321:355-68. doi: 10.1113/jphysiol.1981.sp013989. PMID: 7338816; PMCID: PMC1249631.
- ↑ Jump up to: 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 Liu CY, Arteaga AC, Fung SE, Cortina MS, Leyngold IM, Aakalu VK. Corneal neurotization for neurotrophic keratopathy: Review of surgical techniques and outcomes. Ocul Surf. 2021 Feb 26;20:163-172. doi: 10.1016/j.jtos.2021.02.010. Epub ahead of print. PMID: 33647470.
- ↑ Jump up to: 7.0 7.1 Di Zazzo A, Coassin M, Varacalli G, Galvagno E, De Vincentis A, Bonini S. Neurotrophic keratopathy: Pros and cons of current treatments. Ocul Surf. 2019 Oct;17(4):619-623. doi: 10.1016/j.jtos.2019.09.002. Epub 2019 Sep 14. PMID: 31526824.
- ↑ Jump up to: 8.0 8.1 Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003 Nov;17(8):989-95. doi: 10.1038/sj.eye.6700616. PMID: 14631406.
- ↑ Jump up to: 9.0 9.1 9.2 NaPier E, Camacho M, McDevitt TF, Sweeney AR. Neurotrophic keratopathy: current challenges and future prospects. Ann Med. 2022 Dec;54(1):666-673. doi: 10.1080/07853890.2022.2045035. PMID: 35243932; PMCID: PMC8903790.
- ↑ Jump up to: 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009 Jan;123(1):112-20.
- ↑ Jump up to: 11.0 11.1 Elsberg CA. Experiments on motor nerve regeneration and direct neurotization of paralyzed muscles by their own and by foreign nerves. Science. 1917 Mar 30;45(1161):318-20.
- ↑ Maldonado AA, Bishop AT, Spinner RJ, Shin AY. Five Operations That Give the Best Results after Brachial Plexus Injury. Plast Reconstr Surg. 2017 Sep;140(3):545-556.
- ↑ Jump up to: 13.0 13.1 Samii M. Autologe Nerven-Transplantation im Trigeminusbereich. Med Mitt. 1972;46:189–94.
- ↑ Korus L, Ross DC, Doherty CD, Miller TA. Nerve transfers and neurotization in peripheral nerve injury, from surgery to rehabilitation. J Neurol Neurosurg Psychiatry. 2016 Feb;87(2):188-97.
- ↑ Garcia RM, Hadlock TA, Klebuc MJ, Simpson RL, Zenn MR, Marcus JR. Contemporary solutions for the treatment of facial nerve paralysis. Plast Reconstr Surg. 2015 Jun;135(6):1025e-1046e.
- ↑ Samii M. Reconstruction of the trigeminal nerve. In: Samii M, Jannetta PJ, The cranial nerves. Berlin Heidelberg New York: Springer-Verlag, 1981:352–8.
- ↑ Jump up to: 17.0 17.1 17.2 Wisely CE, Rafailov L, Cypen S, Proia AD, Boehlke CS, Leyngold IM. Clinical and Morphologic Outcomes of Minimally Invasive Direct Corneal Neurotization. Ophthalmic Plast Reconstr Surg. 2020 Sep/Oct;36(5):451-457. doi: 10.1097/IOP.0000000000001586. PMID: 32032169.
- ↑ Lau N, Osborne SF, Vasquez-Perez A, Wilde CL, Manisali M, Jayaram R. Corneal Neurotization Using the Great Auricular Nerve for Bilateral Congenital Trigeminal Anesthesia. Cornea. 2021 Nov 24. doi: 10.1097/ICO.0000000000002951. Epub ahead of print. PMID: 34839333.
- ↑ Rollon-Mayordomo A, Mataix-Albert B, Espejo-Arjona F, Herce-Lopez J, Lledo-Villar L, Caparros-Escudero C, Infante-Cossio P. Neurotrophic Keratitis in a Pediatric Patient With Goldenhar Syndrome and Trigeminal Aplasia Successfully Treated by Corneal Neurotization. Ophthalmic Plast Reconstr Surg. 2021 Nov 16. doi: 10.1097/IOP.0000000000002086. Epub ahead of print. PMID: 34798657.
- ↑ Kim JS, Rafailov L, Leyngold IM. Corneal Neurotization for Postherpetic Neurotrophic Keratopathy: Initial Experience and Clinical Outcomes. Ophthalmic Plast Reconstr Surg. 2021 Jan-Feb 01;37(1):42-50. doi: 10.1097/IOP.0000000000001676. PMID: 32332687.
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 Rosenblatt, T.R., Sears, C.M., Park, J.K. et al. Corneal Neurotization and Novel Medical Therapies for Neurotrophic Keratopathy. Curr Ophthalmol Rep 8, 252–266 (2020). https://doi.org/10.1007/s40135-020-00254-w
- ↑ Feroze KB, Patel BC. Neurotrophic Keratitis. 2022 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 28613758.
- ↑ Fung SSM, Catapano J, Elbaz U, Zuker RM, Borschel GH, Ali A. In Vivo Confocal Microscopy Reveals Corneal Reinnervation After Treatment of Neurotrophic Keratopathy With Corneal Neurotization. Cornea. 2018 Jan;37(1):109-112. doi: 10.1097/ICO.0000000000001315. PMID: 29053558.
- ↑ Jump up to: 24.0 24.1 24.2 24.3 24.4 24.5 Jacinto F, Espana E, Padilla M, et al. Ipsilateral supraorbital nerve transfer in a case of recalcitrant neurotrophic keratopathy with an intact ipsilateral frontal nerve: a novel surgical technique. Am J Ophthalmol Case Rep 2016; 4:14–17.
- ↑ Jump up to: 25.0 25.1 25.2 25.3 25.4 25.5 25.6 Malhotra R, Elalfy MS, Kannan R, Nduka C, Hamada S. Update on corneal neurotisation. Br J Ophthalmol. 2019 Jan;103(1):26-35.
- ↑ Domeshek LF, Hunter DA, Santosa K, Couch SM, Ali A, Borschel GH, Zuker RM, Snyder-Warwick AK. Anatomic characteristics of supraorbital and supratrochlear nerves relevant to their use in corneal neurotization. Eye (Lond). 2019 Mar;33(3):398-403.
- ↑ Jump up to: 27.0 27.1 27.2 27.3 Koaik M, Baig K. Corneal neurotization. Curr Opin Ophthalmol. 2019 Jul;30(4):292-298.
- ↑ Wolkow N, Habib LA, Yoon MK, Freitag SK. Corneal Neurotization: Review of a New Surgical Approach and Its Developments. Semin Ophthalmol. 2019;34(7-8):473-487. doi: 10.1080/08820538.2019.1648692. Epub 2019 Aug 1. PMID: 31370735.
- ↑ Weissler JM, Koltz PF, Carney MJ, Serletti JM, Wu LC. Sifting through the Evidence: A Comprehensive Review and Analysis of Neurotization in Breast Reconstruction. Plast Reconstr Surg. 2018 Mar;141(3):550-565.
- ↑ Jump up to: 30.0 30.1 30.2 30.3 30.4 Leyngold IM, Yen MT, Tian J, Leyngold MM, Vora GK, Weller C. Minimally Invasive Corneal Neurotization With Acellular Nerve Allograft: Surgical Technique and Clinical Outcomes. Ophthalmic Plast Reconstr Surg. 2019 Mar/Apr;35(2):133-140.
- ↑ Jump up to: 31.0 31.1 Leyngold I, Weller C, Leyngold M, Tabor M. Endoscopic corneal neurotization: technique and initial experience. Ophthalmic Plast Reconstr Surg 2018; 34:82–85.
- ↑ Jump up to: 32.0 32.1 Elbaz U, Bains R, Zuker RM, et al. Restoration of corneal sensation with regional nerve transfers and nerve grafts. JAMA Ophthalmol 2014; 132:1289.
- ↑ Jump up to: 33.0 33.1 Bains RD, Elbaz U, Zuker RM, Ali A, Borschel GH. Corneal neurotization from the supratrochlear nerve with sural nerve grafts: a minimally invasive approach. Plast Reconstr Surg. 2015 Feb;135(2):397e-400e. doi: 10.1097/PRS.0000000000000994. PMID: 25626824.
- ↑ Benkhatar H, Levy O, Goemaere I, Borderie V, Laroche L, Bouheraoua N. Corneal Neurotization With a Great Auricular Nerve Graft: Effective Reinnervation Demonstrated by In Vivo Confocal Microscopy. Cornea. 2018 May;37(5):647-650. doi: 10.1097/ICO.0000000000001549. PMID: 29474300.
- ↑ Jump up to: 35.0 35.1 Jowett N, Pineda Ii R. Corneal neurotisation by great auricular nerve transfer and scleral-corneal tunnel incisions for neurotrophic keratopathy. Br J Ophthalmol. 2019 Sep;103(9):1235-1238. doi: 10.1136/bjophthalmol-2018-312563. Epub 2018 Nov 23. PMID: 30470713.
- ↑ Ting DSJ, Figueiredo GS, Henein C, et al. Corneal neurotization for neurotrophic keratopathy: clinical outcomes and in vivo confocal microscopic and histopathological findings. Cornea 2018; 37:641–646.
- ↑ Catapano J#1,2,3, Fung SSM#3,4,5, Halliday W3,6, Jobst C2, Cheyne D2, Ho ES1, Zuker RM1,3, Borschel GH1,2,3, Ali A7,4. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: long-term clinical outcomes and evidence of corneal reinnervation. Br J Ophthalmol. 2019 Dec;103(12):1724-1731. doi: 10.1136/bjophthalmol-2018-313042. Epub 2019 Feb 15.
- ↑ Sweeney AR, Wang M, Weller CL, Burkat C, Kossler AL, Lee BW, Yen MT. Outcomes of corneal neurotisation using processed nerve allografts: a multicentre case series. Br J Ophthalmol. 2022 Mar;106(3):326-330. doi: 10.1136/bjophthalmol-2020-317361. Epub 2020 Nov 16. PMID: 33199302.