Cobalamin C Deficiency
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Cobalamin C (cblC) deficiency is an inherited metabolic error causing a wide range of systemic manifestations and severe ophthalmological features, usually presenting as an infantile-onset macular and retinal degeneration, leading to extreme visual acuity loss early in life.
Disease Entity
Disease
Cobalamin C (cblC) deficiency (OMIM #277400, Methylmalonic aciduria and homocystinuria, cblC type) is the most common inborn error of intracellular vitamin B12 metabolism, belonging to the cobalamin (cbl)-related methylation disorders group, along with cblD, cblE, cblF, cblG, cblJ, cblX and MTHFR deficiency. CblC is responsible for a wide range of systemic manifestations and severe ophthalmological features, usually presenting as an infantile-onset macular and retinal degeneration, leading to extreme visual acuity loss early in life.
Epidemiology
Newborn screening (NBS) studies revealed an incidence of 1:100,000 in New York State,[1] 1:121,000 in the state of New Jersey,[2] 1:67,000 in California and 1:46,000 in Hispanic populations,[3] 1:85,000 in Portugal,[4], 1:3,920 in Shandong, China,[5] confirming that cblC deficiency is panethnic and the most common inborn error of intracellular cobalamin metabolism, comprising around 80% of such cases. Some series have shown a predominance of male patients.[6][7][8]
Genetics
Genetic and molecular base
CblC disease is an autosomal recessive disorder caused by mutations in the MMACHC gene (OMIM *609831) on chromosome 1p34.2.[9] The MMACHC gene is responsible for coding the MMACHC protein, which is directly involved in the early cobalamin processing pathway via both cytosol and mitochondrial routes.[10][11] The MMACHC protein is able to bind to cbl-R cargo as it exits the lysosomal compartment and to release R groups by reductive decyanation (in case of cyanocobalamin) and dealkylation (in the case of methylcobalamin and adenosylcobalamin) in the cytosol, resulting in a common cbl intermediate, allowing it to be further processed towards the two final reactions mediated by methymalonyl-CoA-mutase (mitochondrial route) and methionine synthase (cytosolic route) to synthesize the two active cofactors, methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl).[10][12][13] MMACHC is also thought to closely interact with the MMADHC protein in this pathway.[14][15] In absence of normal MMACHC function, intracellular trafficking of cbl is compromised, resulting in increased levels of methylmalonic acid (MMA) and homocysteine (Hcy), as well as decreased production of methionine (Met). An epigenetic cause of cblC disease has recently been described, named epi-cblC, in which a PDRX1 mutation resulting in a MMACHC epimutation causes promoter hypermethylation and thus silences a wild-type MMACHC allele, which causes disease in a patient carrying a single mutant MMACHC allele.[16]
Common mutations and genotype-phenotype correlations
More than 80 MMACHC gene mutations have been described, the most frequent being the c.271dupA mutation, identified in approximately 40-61% of disease alleles.[6][7][8][9] Homozygous c.271dupA is most commonly identified in patients of European descent, is almost universally associated with early-onset disease (< 1 year of age)[4][6][7][17][18] and with the most severe ocular phenotype.[19][20][21][22] The c.331C>T mutation, present in about 5% to 9% of disease alleles, associated with French Canadian, Acadian or Cajun descent, is also associated to early-onset disease when in homozygosity and when in compound heterozygosity with c.271dupA.[6][9][17][23]
The most prevalent mutation in late-onset cases (> 1 year of age) is the homozygous c.394C>T mutation (present in about 20% of all disease alleles), most commonly seen in patients from Indian, Pakistani and Middle Eastern descent, but also detected in Portugal and Italy.[4][6][9][17] Homozygous c.482G>A and compound heterozygotes for c.394C>T mutations are also associated with late-onset disease.[6][9][24] The c.271dupA is common in late-onset cases when in compound heterozygosity with other late-onset-associated alleles.[24][25] In compound heterozygote genotypes, age of onset is harder to predict but usually the less severe mutation tends to attenuate the effect of the more severe one. Compound heterozygotes for c.271dupA and c.394C>T mutations tend to exhibit a phenotype intermediate between early and late-onset disease.[23] Allele expression and MMACHC transcript levels are significantly lower in c.271dupA and c.331C>T mutations compared to normal alleles and c.394C>T alleles, possibly accounting for phenotypical differences.[17] In East Asian children, particularly from Chinese descent, the most common variant found is c.609G > A, comprising around 48-55% of disease alleles in this population. This mutation is more prone to causing early-onset disease in homozygosity.[26][27][28]
Pathophysiology
Overview
During the intracellular metabolic processing of vitamin B12 several complementation groups (cblA-G and mut) intervene in order to synthesize methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl), which are co-factors for the enzymes methylmalonyl-CoA mutase and methionine synthase, respectively. The cblC complementation group intervenes early in this pathway by releasing R groups in the cytosol, after Cbl-R is released from transcobalamin (TC) in the lysosome. Failure in this step, in cblC disease, creates a metabolic block, resulting in downstream build-up of MMA, Hcy, which in turn decreases Met synthesis.[29] Accordingly, the metabolically descriptive name of cblC disease is combined methylmalonic aciduria and homocystinuria with hypomethioninemia.
Metabolic factors
Some clinical aspects of the disease can be attributed, at least in part, to the aforementioned metabolic imbalances. For example, methylcobalamin (MeCbl) levels in cblC are justifiably low, which impairs DNA synthesis and begets the development of megaloblastic anemia.
Homocysteine (Hcy)
Elevated Hcy levels have been associated with cognitive impairment, forms of dementia and with vascular and cerebrovascular disease, in which an Hcy-induced endothelial dysfunction, prothrombotic state and decreased fibrinolysis may play a significant part.[29][30] Indeed, in cblC disease Hcy levels are an established independent risk for vascular disease, including thrombosis, and there seems to be a correlation between Hcy levels and the severity of vascular complications.[31][32] However, the frequency of small vessel disease in cblC patients such as thrombotic microangiopathy (TMA) or, more specifically, hemolytic uremic syndrome (HUS), cannot be solely attributed to high levels of Hcy given that TMA/HUS are not associated with classical homocystinuria (cystathionine-beta synthase deficiency), a disease causing high Hcy and Met levels.[25][33] High levels of Hcy in the central nervous system (CNS) have also been linked to the development of seizures and hydrocephalus.[34][35] Homocysteine levels also cannot be solely responsible for cblC-related ocular disease because other causes of homocystinuria such as cobalamin E, G and MTHFR deficiency do not display similar ophthalmological features.[21]
Methionine (Met)
Decreased levels of S-adenosylmethionine (SAM), an important substrate for methylation reactions, may contribute to cognitive decline, demyelination or myelination abnormalities, such as optic atrophy or pallor, often found in cblC patients, particularly in early-onset forms,[29][34][35][36][37][38] and may play a role in organogenesis and heart development through imbalances of DNA and histone methylation processes.[39] The high rate of congenital anomalies seen in cblC disease points towards a role by the MMACHC gene, protein or resulting metabolic disturbance in fetal and organ development. Methionine also plays a part in formation of reduced glutathione (GSH), which protects the retinal pigmented epithelium (RPE) from oxidative damage, partially explaining the characteristic degeneration of the RPE seen in the retina of cblC patients.[40][41] In cblC disease, glutathione pool (both in reduced and oxidized forms) is decreased and reactive oxygen species (ROS) as well as MnSOD (a marker of mitochondrial ROS) levels are increased. These ROS levels in cblC cultured fibroblasts decrease with OHCbl treatment.[18][42] These results suggest that oxidative stress and damage may play a part in the pathogenesis of cblC. Moreover, methionine normalization in CblC defect has been shown to rescue photoreceptor response, implying a direct role for Met in the normal functioning retina.[43] However, other cobalamin deficiencies (such as CblG) have low Met levels and do not display significant ocular disease, meaning that Met levels are probably contributive but not directly causative in this respect.[21]
Methylmalonic acid (MMA)
The accumulation of organic acids in the brain, such as MMA, may cause direct neurotoxicity, a phenomenon called “metabolic stroke”, and may play a role in acute encephalopathic features and long-term neurotoxicity due to a “trapping” mechanism.[29][44]
Non-metabolic factors
Most attempts of elucidating the pathogenesis of cblC disease have resulted in clinical and biochemical associations which, although promising, still have failed to explain most of the clinical picture of CblC deficiency, and it is likely that many mechanisms work synergistically to that effect.[29] Other hypothesis have focused on alternative molecular pathways. It has been shown that cblC fibroblast cell lines have impaired expression of several proteins important in cytoskeleton assembly and reorganization, signaling and cellular detoxification, as well as some nervous system proteins implicated in neurological diseases.[45] Proteins involved in intracellular trafficking, protein folding, energy metabolism, cytoskeleton organization and assembly were also found to be deregulated and some protein changes are tissue-specific, which may play a role in the pathophysiology of cblC.[46] These findings stress the likelihood that the full phenotype of cblC disease is dependent not only on directly caused metabolic imbalances but also on other still poorly characterized functions of the MMACHC protein and its interactions with other components of cellular metabolic machinery. It is also postulated that polymorphisms in other genes associated with cobalamin metabolism may modify the clinical presentation of cblC disease, as shown in the recently described case of PDRX1 mutations causing MMACHC epimutations with a clear pathogenic influence.[16][23]
Clinical picture
Classification
Two classical phenotypes are described and help classify the disease. Early-onset is the most common form, representing 86-88% of cases. It is defined as disease presenting within the first year of life. Late-onset disease presents after the first year of life, although some authors define late-onset as presentation after four years of age. A prenatal presentation is also described, but it is usually grouped within early-onset cases. These two classical phenotypes also differ substantially in systemic manifestations, neurological impairment, ophthalmological picture and in long-term outcome.[7][47][48]
Systemic manifestations
Prenatal findings
When a prenatal presentation is detected, typical findings consist of intrauterine growth retardation, dysmorphic features, microcephaly and occasionally congenital heart disease such as fetal dilated cardiomyopathy or left ventricular non-compaction.[6][21][49][50][51][52][53][54]
Early-onset disease
Early-onset disease is often severe and, particularly in a setting lacking newborn screening and the rapid institution of treatment, typically manifests acutely as acidosis, hypotonia, failure to thrive, feeding difficulties, microcephaly, seizures, severe ocular disease and hematological abnormalities, such as thrombocytopenia, macrocytic anemia and/or megaloblastic bone marrow, hyper-segmented neutrophils and leukopenia. Short- and long-term neurological manifestations frequently include developmental delay, cognitive impairment, and behavioral disturbances (such as hyperactivity or autistic traits), among others. One paramount feature of early-onset cblC disease is ophthalmological disease, discussed later.[2][5][6][7][8][38][47][55][56][57]
Other relevant and common manifestations of early-onset disease include cardiomyopathy, renal TMA/HUS, glomerulopathy and pulmonary hypertension.[6][7][8][33][39][55][58][59][60] Vascular complications can contribute to early mortality and include recurrent venous thrombosis, pulmonary thrombosis and cerebrovascular disease.[8][32] Demyelinating neuropathy, subacute degeneration of the spinal cord, atrophic gastritis, liver disease, cor pulmonale and protein-losing enteropathy are less frequent complications.[8][50][53][55][59][61][62][63]
Late-onset disease
In late-onset disease, presenting in childhood or adolescence, neurological and psychiatric disturbances are predominant.[6][7][8][47][64] Some of these include seizures, corticospinal tract signs, subacute combined degeneration of the spinal cord, peripheral neuropathy, ataxia, dysarthria, progressive encephalopathy, cognitive impairment, dementia, hallucinations and delirium, psychosis, acute mental confusion, depression and bipolar disorder.[25][31][65][66][67][68][69][70][71] Thromboembolic complications can also occur in a significant portion of cases and can heavily contribute to morbidity and mortality.[25][31][70] Other manifestations include nephropathy, combined renal TMA and pulmonary hypertension (rTMA/PAH), HUS, left ventricular hypertrophy and diffuse lung disease.[8][25][31][70][72][73][74][75][76][77][78][79] It should be noted that in late-onset cases, if defined as presenting after 1 year of age, young children present often with rTMA/PAH/HUS, as opposed to older children, adolescent and adults, in which neurological and psychiatric symptoms dominate, as well as thromboembolic complications and glomerulopathies.[25] Long-term outcome in early-onset cases is unfavorable despite treatment, with significant morbidity, and mortality between 11% and 30%, while late-onset cases have more favorable outcome and seldom display ocular disease.[7][8][21][24][47]
Neuro-radiological findings
Magnetic resonance imaging (MRI) findings can include delayed myelination, cortical and corpus callosum atrophy, white matter abnormalities, basal ganglia lesions, hydrocephalus, shortening of the pons. These findings are typically more severe in early-onset cases.[7][19][34][35][37][38][50][55][56] The type and degree of neuroradiological anomalies does not seem to be correlated with the degree of biochemical findings or be able to accurately predict neurological outcome. However, some have found that corpus callosum atrophy and white matter abnormalities tend to be associated with impaired neurological function.[55][56] Nonetheless, although some neurological abnormalities can affect visual performance, there appears to be no strict correlation between MRI findings and visual performance.[35] In late-onset cblC disease, the main MRI findings consist of cortical, cerebellar and spinal atrophy, deep white matter abnormalities, corpus callosum atrophy or agenesis and hydrocephalus.[31][64][65][69][70]
Ophthalmological manifestations
Overview
Ocular disease is one of the most common and impairing traits of early-onset CblC disease, while late-onset patients seldom display any significant ophthalmological involvement.[7][47][80][81] Only two accounts of significant retinal disease in late-onset CblC patients have been described: one case of pigmentary retinopathy and one of adult-onset non-syndromic bull’s eye maculopathy.[47][82] The c.271dupA allele is not only the most common CblC allele in cblC disease but is also almost always associated with a severe ocular phenotype.[19][20][21][22][41] Although the ophthalmological phenotype can vary among early-onset patients, visual function and acuity decrease is often fast and dramatic in early-onset patients, overwhelmingly progressing to legal blindness within the first decade of life and almost universally so in the second decade, despite adequate treatment.[2][19][21][22][32][81][83] Although treatment has a proven positive effect on metabolic control, no clear correlation has been found between the degree of metabolic control and the pace, chronicity and severity of ophthalmological manifestations.[21][84] This resistance to treatment and inexorably progressive nature of ocular disease has a definitive impact on the global picture of early-onset patient, contributing greatly to overall impairment. It has been shown that patients with severe visual or neurovisual impairment had the most intellectual disability, and conversely, the ones with the best intellectual performances had little or no visual symptoms.[56] Furthermore, a correlation has been found between the presence of ocular disease at diagnosis and higher severity scores at follow-up.[7]
Signs and symptoms
Early-onset cblC patients can display signs of ocular disease very early in the course of the disease. The most frequent signs in early stages are abnormal fixation, strabismus and, even more commonly, nystagmus.[19][20][21][22][49][84][85][86][87][88] Nystagmus is estimated to be present in about 70-76% of early-onset cases, can be detected as early as at the time of birth and is most frequently horizontal, high-amplitude and low-velocity.[20][21][40][41][83][86][87][89] The prevalence of strabismus is more variable, estimated to be between 23 and 62%.[7][21][41]
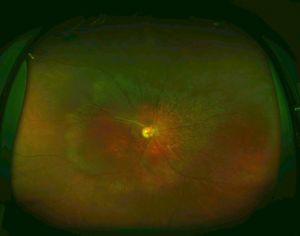
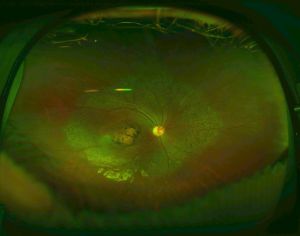
Maculopathy is perhaps the most striking and impairing feature of ocular disease in early-onset CblC disease. Macular changes can develop as early as 35 days of age, often as subtle macular pigmentary or atrophic changes.[90] These macular changes may progress to severe macular lesions in a dramatically fast pace compared to other inherited maculopathies, usually within the first two years of age but as early as 6 months of age.[20][84][88] Maculopathy is estimated to affect 33% of patients before 1 year of age, 60% at 4 years, and 75-86% of early-onset cases overall.[2][21][41] Severe macular lesions may take the form of chorioretinal atrophy or a bull’s eye maculopathy (a hypopigmented perimacular zone surrounded by a hyperpigmented ring), which can progress to a more atrophic macular appearance.[19][20][21][22][40][80][83][84][85][88][89] For example, one case report described a patient with normal fundus at 6 months of age followed by punched-out macular chorioretinal atrophy detected less than a year later.[20] Sometimes the base of these severely atrophic central chorioretinal lesions can become depressed forming a coloboma-like (or pseudocolobomatous) lesion, described as early as 6 to 8 months of age.[21][22][80][84] A posterior staphyloma superimposed on a coloboma-like lesion has also been described.[20]
In addition to maculopathy, the surrounding retina is also commonly affected, although at an apparently lower rate and slower pace. In these cases of more generalized pigmentary retinopathy, macular changes begin expanding outwards, involving the mid-peripheral retina and perivascular retinal regions, creating a granular, “salt-and-pepper” or “bone-spicule” pattern, sometimes in a geographic distribution, which can ultimately result in end-stage retinal degeneration, usually early in the second decade of life, culminating in legal blindness.[19][20][21][22][49][80][83][84][85][88]
The macular changes described appear during a critical period of postnatal foveal development, in which photoreceptors and ganglion cells may be especially susceptible to damage in cblC disease, metabolic or otherwise.[80][84] One case of normal OCT imaging at 5 days old followed by overt retinal changes at 8 months old supports this claim.[22] Some studies suggest that these early changes may begin antenatally, but prenatal treatment does not seem to effectively prevent retinal disease, although it may delay its onset and progression in some cases.[20][21][89][91][92] Recent cases illustrated that the institution of prenatal treatment in a younger affected sibling of another affected patient can result in better visual function and less severe retinal findings, as well as improving overall clinical picture.[20][21][92] The retinal disease seen in cblC may also be part of a degenerative process, owing to the progressive decline in ERG amplitudes over time and to the relative lack of efficacy of prenatal treatment, but it is likely that both developmental and degenerative hypothesis have their merits.[40]
Optic pallor or atrophy can coexist with retinal disease or can be an isolated finding. The atrophy can be diffuse or predominantly temporal.[19][20][21][40][83][84][86][88][89][90] Vascular changes are also a frequent accompanying finding, ranging from increased tortuosity to vascular thinning or attenuation.[20][21][40][49][90]
Visual performance and visual acuity (VA) evaluation may be challenging in younger patients and several methods and tools can be employed according to the age and overall neurodevelopmental stage of the patient. Most early-onset cblC patients suffer from decreased VA, with more than half being moderately to severely impaired (logMAR > 0.7) by 7 years of age in one review of literature and averaging 0.9 and 1.4 logMAR in groups aged 5 to 10 and 10 to 15 years old, respectively, in a more recent series.[21][41]
Ancillary testing
Fundus autofluorescence (FAF)
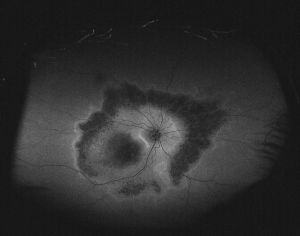
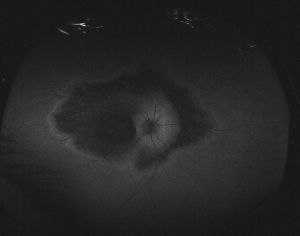
The process of outward expansion of macular lesions is often best characterized with the aid of FAF imaging, which can be a valuable tool in detailing macular and retinal changes. Typical images include a sparing of peripheral retinal fields surrounding mid-peripheral areas of variable hypo- and hyper-autofluorescence. The macular region is hypoautofluorescent (characteristic of atrophic regions) and is surrounded by perimacular ring of hyperautofluorescence (owing to photoreceptor degeneration), indicating progressive and expanding geographical atrophy. It is of note that the peripapillary regions are often spared, as is the case in other retinal degenerations such as in Stargardt disease, and in correlation with OCT findings described below.[20][41]
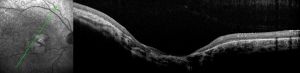
Optical coherence tomography (OCT)
Retinal changes can be evaluated in detail with OCT imaging. The most frequent finding is retinal thinning, particularly and earliest in the foveal region, This can be detected as early as 4 months old, presumably owing to cone loss.[21][22][84][88] Ganglion cell layer (GCL) also shows early and characteristic thinning in parafoveal areas, accompanied by outer nuclear layer (ONL) and retinal nerve fiber layer (RNFL) thinning, although the GCL seems to show greater susceptibility to damage than photoreceptors.[22][41][80][84] Disruption of outer retinal layers with photoreceptor OS and ONL losses are also early findings in parafoveal and peripheral areas.[22][84] Moreover, early deposition of hyperreflective material or debris can be detected anterior to the RPE in the macular region, presumably due to macrophageal activity, which may indicate that the primary changes are in the deeper retinal layers.[20][22] In severe atrophic regions, clear architectural decay due to lamellar loss is seen, with amalgamation of INL and ONL layers and thinning of the RFNL. The INL is often initially spared even in the presence of severe photoreceptor loss but as central atrophy progresses, the inner retinal remodeling causes both INL and RFNL to become thickened. In peripapillary areas the RPE and the overall lamellar architecture is better preserved, particularly on the nasal side, but the RFNL is thickened and ONL and GCL losses are clearly present on the temporal side. Thickening of the RFNL is likely due to the replacement of nerve fibers with non-neuronal elements as part of a remodeling response or gliosis.[80][84] Bull’s eye maculopathy in OCT imaging is characterized by a sharp transition between well preserved peripheral retinal architecture and a zone of GCL and ONL thinning and parafoveal atrophy of outer retinal structures, accompanied by posterior backscattering.[20][84] Despite pigmentary changes being a common finding in the fundus of cblC patients, the initial preservation of the RPE layer in the presence of ONL and GCL loss indicates that the RPE changes seen later on may be secondary to photoreceptor loss.[84] In the adult-onset non-syndromic bull’s eye maculopathy case described earlier, OCT imaging did not show significant GCL loss in the presence of marked foveal thinning, suggesting lesser GCL vulnerability later in life.[82]
Electrophysiology
Electroretinography (ERG) can be normal but more frequently yields variable but progressive decrease and delay of photopic and scotopic responses, reflecting non-selective photoreceptor dysfunction and generalized retinal degeneration.[20][22][40][83][84][88][89] Although ERG can arguably correlate better with visual performance than fundus findings, no clear correlation has yet been established.[19][21][41][83][84] Visual evoked potentials (VEP) often reveal primitive, disorganized and reduced amplitudes in VEP responses, ranging from no response at all to normal response in some cases.[86][88]
Pathology
A clinicopathological correlate can be drawn, with histological analysis showing abnormal pigmentation of the RPE, photoreceptor loss, ONL degeneration, loss of RFNL and ganglion cells in the region of the maculopapillary bundle and optic atrophy.[90]
Diagnosis
Diagnostic approach
Upon suspicion of a vitamin B12 metabolic disease or remethylation disorder, it is reasonable to start a diagnostic approach by measuring total plasma homocysteine and plasmatic MMA levels, which if elevated, should prompt evaluation of MMA related metabolites, plasma methionine and an acylcarnitine profile, preferably before treatment is initiated. CblC patients present with elevated MMA and total Hcy (tHcy) levels and usually also with low plasma methionine, normal serum B12 and folate levels and a variably abnormal acylcarnitine profile.[48][81][93] These biochemical features can also be found in cblD, cblF, cblJ diseases, but these are rarer conditions.
Before the discovery of the MMACHC gene, after cblC or other inborn error of B12 metabolism was suspected, complementation analysis with cultured fibroblasts would have been the following step. Nowadays, because modern genetic sequencing can provide rapid and reliable results, MMACHC sequencing is the preferred diagnostic method after clinical and biochemical data point towards methylmalonic aciduria with homocystinuria.
Newborn screening
Newborn screening (NBS) for cblC disease is strongly recommended because it allows for for rapid diagnosis and prompt initiation of treatment, which can have definitive impact on early and late morbidity and mortality.[2][48][81][94] NBS for disorders of metabolic diseases such as propionate metabolism disorders, organic acidurias and cobalamin defects is now routine in several countries thanks to the introduction of tandem mass spectrometry on dried blood spot samples.[95] Moderate elevations of C3-carnitine (propionylcarnitine) are common but nonspecific in cblC cases. The use of C3 levels in conjunction with ratios between C3 and other carnitines (such as C3/C2, C3/C0), C3/methionine ratio and methionine levels may be helpful in screening newborns for this condition.[1][4][60][81][94][96][97] Second tier strategies such as measurement of urinary organic acids (such as MMA) and of plasma MMA and Hcy have been proposed.[94][98] The inclusion of C17 (heptadecanoylcarnitine) as a first tier marker in MMA/PA and cblC has also been suggested.[81][99] The typical NBS profile of a cblC patient is a moderate elevation of C3 (5-10 µmol/L), increased C3:C2 ratio (> 0.2) along with low methionine and increased C3/Met ratio, although some cases described were missed by NBS, which seems more likely to happen in patients presenting with a milder/late-onset mutation and low carnitine levels.[3][4][24][98][100]
With the growing implementation of cbC disease NBS programs, it is likely that the two phenotypical groups, early and late-onset, may become blurred with time, since NBS does not differentiate between children who would have presented symptoms within the first year of life from those who would have presented with the disease later. Therefore, positive NBS cases, regardless of their mutation and its associations with classical phenotypical presentations, should undergo clinical and neuropsychological evaluations to account for the heterogeneous spectrum of manifestations these patients may develop.[67]
Prenatal diagnosis
Prenatal diagnosis of cblC disease can be done through measurements of MMA, tHcy and C3 in the amniotic fluid, complementation studies in cultured amniocytes or villous cells or, more recently, through gene sequencing from chorionic villus and amniotic fluid samples.[81][91][101][102] Non-invasive prenatal diagnosis using target region sequencing of cell-free DNA in maternal plasma has also been reported.[103] Diagnosing cblC disease during a pregnancy may allow the parents to terminate an affected pregnancy or help them prepare for the birth of another affected child, and allows for institution of prenatal treatment or soon after birth.[101] Prenatal treatment is effective in preventing acute metabolic decompensation, may halt the development of several systemic manifestations and may even result in less cognitive dysfunction and better visual function.[20][21][91] A case published by Trefz et al. describes a patient that received 10mg of OHCbl thrice weekly and 5mg/day of folic acid from the 15th week of gestation, which showed good metabolic control, normal cognitive development and no ocular involvement, compared to her sibling who shared the same early-onset prone genotype and was treated only postnatally.[92] In a previous case published by Huemer et al., a lower dose of OHCbl was used and prenatal treatment was initiated at 24 weeks of gestation, and the child had an uneventful birth and did not suffer from acute metabolic decompensation or severe systemic manifestations but went on to develop cognitive impairment and ocular disease.[91] Thus, prenatal diagnosis may present a precious opportunity to implement treatment that may have significant impact on early and late morbidity and mortality.
Differential diagnosis
Differential diagnosis should include:
- Stargardt's disease
- Congenital Zika syndrome
- Best disease
- TORCH complex maculopathies (ie, toxoplasmosis)
- Other cobalamin deficiencies
Management
Overview and proposed protocol
The goal of treatment of cblC disease is to improve metabolic control, reduce manifestations and complications of the disease and finally to reduce morbimortality. In spite of treatment practices varying widely between centers, a combined approach is used most often, consisting mainly of parenteral hydroxocobalamin and betaine supplementation, both of which have been proven to be effective.[8][48][55][81] Additional folic or folinic acid and carnitine supplementation have been used, although definitive evidence for their clinical effect is still lacking.[48][81][104][105]
A proposed treatment protocol would include:
- Hydroxocobalamin (parenteral) – 1mg/day (starting dose) followed by individual titration
- Betaine (oral) – 250mg/kg, divided in 2-3 doses
- Folic/folinic acid (oral) – 5-15mg/day divided in 2-3 doses
- Levocarnitine (oral) – between 50 and 200 mg/kg/day
Hydroxocobalamin (OHCbl)
Empiric treatment of cblC disease with daily vitamin B12 follows the premise that megadose vitamin therapy may overcome the metabolic defect. CblC patients are unresponsive to CNCbl but respond clinically and metabolically to OHCbl therapy, possibly because the defective MMACHC protein is unable to bind to and decyanate CNCbl, preventing it from being further processed.[12][13][47][104][106][107][108] Futhermore, it should be underlined that oral OHCbl is not as effective as parenteral treatment.[104] OHCbl is effective in in lowering MMA and tHcy and increasing Met levels in a dose-dependent fashion, and OHCbl dose escalation may improve metabolic control in patients with difficult metabolic response to treatment.[48][81][109][110][111] Moreover, reducing the frequency of administration can lead to sub-therapeutic levels, worsening metabolic control and neurological deterioration.[48][112] Currently, the proposed regimen recommends a starting dose of 1 mg daily intramuscular injection of OHCbl – which in a neonatal setting usually corresponds to about 0.3mg/kg – with subsequent titration according to metabolic control, and once a favorable and stable metabolic status is reached, doses should be maintained, avoiding the temptation to further increase the dose with the hope of completely normalizing metabolite levels, which is especially hard in the case of tHcy. Complete normalization of metabolic parameters is rare, but more common in late-onset cases.[21][48][81][111][113]
Betaine
Betaine treatment, which has been shown to work synergistically with OHCbl, further helps in lowering Hcy levels and increasing Met levels by acting on the conversion of Hcy to Met via the betaine-homocysteine-S-methyltransferase pathway, which does not require vitamin B12 as a cofactor and is presumed to account for up to 50% of homocysteine remethylation.[29][104][113] An anhydrous betaine powder formulation is preferred, the recommended daily oral dose being 250mg/kg, usually divided in 2 to 3 doses.[48][81] Both OHCbl and betaine seem to be safe, well tolerated and have little to no side effects even in much higher doses than recommended, and both have been used safely during pregnancy.[21][48][91][109][114]
Folates
Folic or folinic acid – the latter may be preferred because it is a more stable form of the vitamin and it crosses the blood brain barrier more efficiently – given in 2-3 doses of daily 5-15mg may theoretically be helpful by bypassing the “folate trap” created by decreased methionine-synthase activity and as adjunct to betaine metabolism, but its clinical value is not proven.[48][81][104][105][113]
Other therapeutic options
Levocarnitine is used as an adjuvant in increasing the excretion of organic acids such as methylmalonate and in preventing carnitine deficiency but its clinical role in cblC has not been proven. L-methionine supplementation may also be used in some selected cases if low Met levels persist despite adequate treatment and should be adequately titrated.[81][113]
The use of protein restriction or medical foods in some organic acidurias such as isolated methylmalonic aciduria or propionic acidemia (MMA/PA) is indicated to reduce the build-up of deleterious organic acids. However, in cblC disease, MMA levels can usually be controlled by an adequate intake of parenteral OHCbl. The use of protein restriction and/or methionine-poor MMA/PA medical foods, despite being able to further decrease MMA levels, may accentuate systemic and CNS hypomethioninemia and other amino acid imbalances. Its use correlates with lower height-for-age and head circumference z-scores and may be detrimental to overall clinical picture, and thus is strongly contraindicated in the treatment of cblC patients.[2][48][81][115]
The relevance of early treatment
Early and adequate treatment and metabolic control has been shown to reduce mortality, improve growth parameters and global clinical picture, especially concerning systemic non-neurological and non-ophthalmological manifestations, such as acute metabolic acidosis, anemia, hypotonia, lethargy, seizures, hemolytic-uremic syndrome, cardiomyopathy and vascular complications.[7][8][39][47][48][51][55][81] Although it is difficult to achieve complete metabolic control, it should be stressed that higher mean Hcy levels are correlated with more severe neurologic impairment, higher severity scores during follow-up in early-onset cases and with overall clinical impression in late-onset cases, and Met levels are negatively correlated with overall clinical impression in both early and late-onset groups.[7] Moreover, it has been observed that 100% of the lowest lifetime Met and 75% of the peak Hcy levels occur in the neonatal period.[2] Given that neurodevelopmental outcomes such as language development are correlated with an individual’s lowest Met level, highest peak Hcy and mean lifetime total Hcy, it becomes clear that early treatment and metabolic control is paramount.[2][67]
Furthermore, clinically detected disease often shows worse neurological impairment and manifestations as opposed to pre-clinically detected disease, which may indicate that early detection and treatment can be particularly helpful in preventing acute neurological disease in the neonatal/early infantile period.[8] Despite the improvement of some neurological manifestations, cognitive impairment and development delay seem to be resistant to treatment in early-onset patients and often accompany severe multi-system disease and visual disability, factors which in conjunction heavily determine the unfavorable long-term outcome of these patients.[5][7][31][38][56][69][110][112]
The ophthalmological involvement in CblC disease is notoriously resistant to treatment. Some evidence suggests that early treatment may attenuate or delay the appearance of retinopathy, but a recent series showed that even with the institution of early treatment in NBS-positive patients, all presented at the first ophthalmological evaluation with maculopathy and/or reduced visual acuity.[7][21][88]
Since myelination of CNS and retinal maturation all occur during the last trimester of pregnancy and first year of life, it is reasonable to propose the initiation of prenatal treatment whenever possible.[22] Prenatal treatment seems capable of preventing acute early decompensation, achieve early metabolic control and delaying some neurological symptoms. Nonetheless, it seems unable to effectively prevent the development of most ophthalmological manifestations, although it has been shown to at least delay its onset and slow its progression.[20][21][81][86][91][92]
Treatment in late-onset disease
In the case of late-onset patients, treatment is not only able to resolve and prevent most systemic manifestations such as thromboembolic complications, rTMA/PAH/HUS, but has also been shown to improve psychiatric disturbances, cognitive impairment, corticospinal tract signs and myelopathy/neuropathy signs, which contributes to the better long-term outcome of these subset of patients.[25][31][33][58][59][64][65][68][69][70][71][75][77][79][81]
Future therapeutic avenues
There is an indisputable unmet need for alternative therapeutic targets and approaches to cblC disease in order to deal with the most treatment resistant complications of the disease. New antioxidant compounds, novel local forms of treatment delivery and gene transfer approaches may play a part in of cblC treatment.[21][41] Recently, Venditti et al. submitted a patent application (WO2017099838A1, PCT/US2016/029512) for a synthetic MMACHC polynucleotide intended to be used for gene therapy in the future.
Follow-up and surveillance strategies
Follow-up strategies should include periodic measurements of plasma or urine MMA, plasma Hcy and Met levels as well as plasma B12 levels in order to confirm that B12 levels achieve the therapeutic range, echocardiographic examination and regular neurological and behavioral assessment.[1][41][48]
It’s highly recommended that any patient with the diagnosis of cblC disease receive an early ophthalmological observation regardless of the age of diagnosis or the severity of the disease, ideally at a tertiary center and by a pediatric ophthalmologist. Early counselling regarding the poor visual outcome and practical resources for the visually impaired is also strongly advised.[41][81]
First ophthalmological observation should be offered at the time of diagnosis, even in the case of neonatal diagnosis, and should be repeated in at least 6 months intervals in the first years of life. It is recommended to perform ancillary testing such as retinal photography, electrophysiology testing and OCT imaging in the first 18 months, whenever it is appropriate and opportune to sedate the child. Such complementary testing not only may be of prognostic value but can also provide a baseline from which to evaluate progression in subsequent times, scheduling these exams every 2 or 3 years. After 2 years of age, spacing of ophthalmological observations is reasonable according to individual disease severity, progression and response to treatment. In the case of late-onset cases and older patients with stable eye disease, 5-year intervals for observation and testing is a reasonable approach.[41]
References
- ↑ 1.0 1.1 1.2 Weisfeld-Adams JD, Morrissey MA, Kirmse BM, et al. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Mol Genet Metab. 2010;99(2):116-123.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Ahrens-Nicklas RC, Whitaker AM, Kaplan P, et al. Efficacy of early treatment in patients with cobalamin C disease identified by newborn screening: A 16-year experience. Genet Med. 2017;19(8):926-935.
- ↑ 3.0 3.1 Cusmano-Ozog K, Lorey F, Levine S, et al. Cobalamin C disease and expanded newborn screening: the California experience. J Investig Med. 2007;55(1):S90.
- ↑ 4.0 4.1 4.2 4.3 4.4 Nogueira C, Marcão A, Sousa C, et al. Molecular picture of cobalamin C/D defects before and after newborn screening era. J Med Screen. 2016;24(1):6-11.
- ↑ 5.0 5.1 5.2 Han B, Cao Z, Tian L, et al. Clinical presentation, gene analysis and outcomes in young patients with early-treated combined methylmalonic acidemia and homocysteinemia (cblC type) in Shandong province, China. Brain Dev. 2016;38(5):491-497.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 Nogueira C, Aiello C, Cerone R, et al. Spectrum of MMACHC mutations in Italian and Portuguese patients with combined methylmalonic aciduria and homocystinuria, cblC type. Mol Genet Metab. 2008;93(4):475-480.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 Fischer S, Huemer M, Baumgartner M, et al. Clinical presentation and outcome in a series of 88 patients with the cblC defect. J Inherit Metab Dis. 2014;37(5):831-840.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 Huemer M, Diodato D, Martinelli D, et al. Phenotype, treatment practice and outcome in the cobalamin-dependent remethylation disorders and MTHFR deficiency: Data from the E-HOD registry. J Inherit Metab Dis. 2019;42(2):333-352.
- ↑ 9.0 9.1 9.2 9.3 9.4 Lerner-Ellis JP, Tirone JC, Pawelek PD, et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat Genet. 2006;38(1):93-100.
- ↑ 10.0 10.1 Froese DS, Gravel RA. Genetic disorders of vitamin B 12 metabolism: Eight complementation groups - Eight genes. Expert Rev Mol Med. 2010;12(November):1-20.
- ↑ Watkins D, Rosenblatt DS. Inborn errors of cobalamin absorption and metabolism. Am J Med Genet Part C Semin Med Genet. 2011;157(1):33-44.
- ↑ 12.0 12.1 Banerjee R. B12 trafficking in mammals: A for coenzyme escort service. ACS Chem Biol. 2006;1(3):149-159.
- ↑ 13.0 13.1 Kim J, Gherasim C, Banerjee R. Decyanation of vitamin B12 by a trafficking chaperone. Proc Natl Acad Sci. 2008;105(38):14551-14554.
- ↑ Plesa M, Kim J, Paquette SG, et al. Interaction between MMACHC and MMADHC, two human proteins participating in intracellular vitamin B12 metabolism. Mol Genet Metab. 2011;102(2):139-148.
- ↑ Froese DS, Kopec J, Fitzpatrick F, et al. Structural insights into the MMACHC-MMADHC protein complex involved in vitamin B12 trafficking. J Biol Chem. 2015;290(49):29167-29177.
- ↑ 16.0 16.1 Guéant JL, Chéry C, Oussalah A, et al. APRDX1 mutant allele causes a MMACHC secondary epimutation in cblC patients. Nat Commun. 2018;9(1):1-12.
- ↑ 17.0 17.1 17.2 17.3 Lerner-Ellis JP, Anastasio N, Liu J, et al. Spectrum of mutations in MMACHC, allelic expression, and Evidence for Genotype-Phenotype Correlations. Hum Mutat. 2009;30(7):1072-1081.
- ↑ 18.0 18.1 Richard E, Jorge-Finnigan A, Garcia-Villoria J, et al. Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency type C ( cblC ) with homocystinuria (MMACHC). Hum Mutat. 2009;30(11):1558-1566.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 19.8 Gizicki R, Robert MC, Gómez-López L, et al. Long-term visual outcome of methylmalonic aciduria and homocystinuria, cobalamin C type. Ophthalmology. 2014;121(1):381-386.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 20.11 20.12 20.13 20.14 20.15 20.16 20.17 20.18 Ku CA, Ng JK, Karr DJ, et al. Spectrum of ocular manifestations in cobalamin C and cobalamin A types of methylmalonic acidemia. Ophthalmic Genet. 2016;37(4):404-414.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 21.13 21.14 21.15 21.16 21.17 21.18 21.19 21.20 21.21 21.22 21.23 21.24 21.25 21.26 21.27 Brooks BP, Thompson AH, Sloan JL, et al. Ophthalmic manifestations and long-term visual outcomes in patients with cobalamin C deficiency. Ophthalmology. 2016;123(3):571-582.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 22.12 22.13 Bacci GM, Donati MA, Pasquini E, et al. Optical coherence tomography morphology and evolution in cblC disease-related maculopathy in a case series of very young patients. Acta Ophthalmol. 2017;95(8):e776-e782.
- ↑ 23.0 23.1 23.2 Morel CF, Lerner-Ellis JP, Rosenblatt DS. Combined methylmalonic aciduria and homocystinuria (cblC): Phenotype-genotype correlations and ethnic-specific observations. Mol Genet Metab. 2006;88(4):315-321.
- ↑ 24.0 24.1 24.2 24.3 Almannai M, Marom R, Divin K, et al. Milder clinical and biochemical phenotypes associated with the c.482G>A (p.Arg161Gln) pathogenic variant in cobalamin C disease: Implications for management and screening. Mol Genet Metab. 2017;122(1-2):60-66.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 Huemer M, Scholl-Bürgi S, Hadaya K, et al. Three new cases of late-onset cblC defect and review of the literature illustrating when to consider inborn errors of metabolism beyond infancy. Orphanet J Rare Dis. 2014;9:161.
- ↑ Liu MY, Yang YL, Chang YC, et al. Mutation spectrum of MMACHC in Chinese patients with combined methylmalonic aciduria and homocystinuria. J Hum Genet. 2010;55(9):621-626.
- ↑ Hu S, Mei S, Liu N, Kong X. Molecular genetic characterization of cblC defects in 126 pedigrees and prenatal genetic diagnosis of pedigrees with combined methylmalonic aciduria and homocystinuria. BMC Med Genet. 2018;19(1):1-7.
- ↑ Wang F, Han L, Yang Y, et al. Clinical, biochemical, and molecular analysis of combined methylmalonic acidemia and hyperhomocysteinemia (cblC type) in China. J Inherit Metab Dis. 2010;33(SUPPL. 3).
- ↑ 29.0 29.1 29.2 29.3 29.4 29.5 Martinelli D, Deodato F, Dionisi-Vici C. Cobalamin C defect: Natural history, pathophysiology, and treatment. J Inherit Metab Dis. 2011;34(1):127-135.
- ↑ Castro R, Rivera I, Blom HJ, Jakobs C, Tavares de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: An overview. J Inherit Metab Dis. 2006;29(1):3-20.
- ↑ 31.0 31.1 31.2 31.3 31.4 31.5 31.6 Thauvin-Robinet C, Roze E, Couvreur G, et al. The adolescent and adult form of cobalamin C disease: Clinical and molecular spectrum. J Neurol Neurosurg Psychiatry. 2008;79(6):725-728.
- ↑ 32.0 32.1 32.2 Carrillo-Carrasco N, Venditti CP, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. II. Complications, pathophysiology, and outcomes. J Inherit Metab Dis. 2012;35(1):103-114.
- ↑ 33.0 33.1 33.2 Lemoine M, François A, Grangé S, et al. Cobalamin C Deficiency Induces a Typical Histopathological Pattern of Renal Arteriolar and Glomerular Thrombotic Microangiopathy. Kidney Int Reports. 2018;3(5):1153-1162.
- ↑ 34.0 34.1 34.2 Rossi A, Cerone R, Biancheri R, et al. Early-onset combined methylmalonic aciduria and homocystinuria: Neuroradiologic findings. Am J Neuroradiol. 2001;22(3):554-563.
- ↑ 35.0 35.1 35.2 35.3 Longo D, Fariello G, Dionisi-Vici C, et al. MRI and 1H-MRS findings in early-onset cobalamin C/D defect. Neuropediatrics. 2005;36(6):366-372.
- ↑ Surtees R. Demyelination and inborn errors of the single carbon transfer pathway. Eur J Pediatr. 2006;157(S2):S118-S121.
- ↑ 37.0 37.1 Biancheri R, Cerone R, Schiaffino M, et al. Cobalamin (Cbl) C/D Deficiency: Clinical, Neurophysiological and Neuroradiologic Findings in 14 Cases. Neuropediatrics. 2001;32(01):14-22.
- ↑ 38.0 38.1 38.2 38.3 Weisfeld-Adams JD, Bender HA, Miley-Åkerstedt A, et al. Neurologic and neurodevelopmental phenotypes in young children with early-treated combined methylmalonic acidemia and homocystinuria, cobalamin C type. Mol Genet Metab. 2013;110(3):241-247.
- ↑ 39.0 39.1 39.2 Profitlich LE, Kirmse B, Wasserstein MP, Diaz GA, Srivastava S. High prevalence of structural heart disease in children with cblC-type methylmalonic aciduria and homocystinuria. Mol Genet Metab. 2009;98(4):344-348.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 Schimel AM, Mets MB. The natural history of retinal degeneration in association with cobalamin C (cbl C) disease. Ophthalmic Genet. 2006;27(1):9-14.
- ↑ 41.00 41.01 41.02 41.03 41.04 41.05 41.06 41.07 41.08 41.09 41.10 41.11 41.12 Weisfeld-Adams JD, McCourt EA, Diaz GA, Oliver SC. Ocular disease in the cobalamin C defect: A review of the literature and a suggested framework for clinical surveillance. Mol Genet Metab. 2015;114(4):537-546.
- ↑ Pastore A, Martinelli D, Piemonte F, et al. Glutathione metabolism in cobalamin deficiency type C (cblC). J Inherit Metab Dis. 2014;37(1):125-129.
- ↑ Tsina EK, Marsden DL, Hansen RM, Fulton AB. Maculopathy and Retinal Degeneration in Cobalamin C Methylmalonic Aciduria and Homocystinuria. Arch Ophthalmol. 2005;123(8):1143.
- ↑ Kölker S, Sauer SW, Hoffman GF, Müller I, Morath MA, Okun JG. Pathogenesis of CNS involvement in disorders of amino and organic acid metabolism. J Inherit Metab Dis. 2008;31(2):194-204.
- ↑ Hannibal L, DiBello PM, Yu M, et al. The MMACHC proteome: Hallmarks of functional cobalamin deficiency in humans. Mol Genet Metab. 2011;103(3):226-239.
- ↑ Caterino M, Pastore A, Strozziero MG, et al. The proteome of cblC defect: in vivo elucidation of altered cellular pathways in humans. J Inherit Metab Dis. 2015;38(5):969-979.
- ↑ 47.0 47.1 47.2 47.3 47.4 47.5 47.6 47.7 Rosenblatt DS, Aspler AL, Shevell MI, Pletcher BA, Fenton WA, Seashore MR. Clinical heterogeneity and prognosis in combined methylmalonic aciduria and homocystinuria (cblC). J Inherit Metab Dis. 1997;20(4):528-538.
- ↑ 48.00 48.01 48.02 48.03 48.04 48.05 48.06 48.07 48.08 48.09 48.10 48.11 48.12 48.13 Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis. 2012;35(1):91-102.
- ↑ 49.0 49.1 49.2 49.3 Robb RM, Dowton SB, Fulton AB, Levy HL. Retinal degeneration in vitamin B12 disorder associated with methylmalonic aciduria and sulfur amino acid abnormalities. Am J Ophthalmol. 1984;97(6):691-696.
- ↑ 50.0 50.1 50.2 Frattini D, Fusco C, Ucchino V, et al. Early Onset Methylmalonic Aciduria and Homocystinuria cblC Type With Demyelinating Neuropathy. Pediatr Neurol. 2010;43(2):135-138.
- ↑ 51.0 51.1 De Bie I, Nizard SDP, Mitchell GA. Fetal dilated cardiomyopathy: an unsuspected presentation of methylmalonic aciduria and hyperhomocystinuria, cblC type. Prenat Diagn. 2009;29:266-270.
- ↑ Cerone R, Schiaffino MC, Caruso U, Lupino S, Gatti R. Minor facial anomalies in combined methylmalonic aciduria and homocystinuria due to a defect in cobalamin metabolism. J Inherit Metab Dis. 1999;22(3):247-250.
- ↑ 53.0 53.1 Smith SE, Kinney HC, Swoboda KJ, Levy HL. Subacute combined degeneration of the spinal cord in cblC disorder despite treatment with B12. Mol Genet Metab. 2006;88(2):138-145.
- ↑ Tanpaiboon P, Sloan JL, Callahan PF, et al. Noncompaction of the Ventricular Myocardium and Hydrops Fetalis in Cobalamin C Disease. In: JIMD Reports. ; 2012:33-38.
- ↑ 55.0 55.1 55.2 55.3 55.4 55.5 55.6 Deodato F, Boenzi S, Rizzo C, Dionisi-Vici C. The clinical picture of early-onset cobalamin c defect (methylmalonic aciduria and homocystinuria). Paediatr Child Health (Oxford). 2008;18(SUPPL. 1):S57-S60.
- ↑ 56.0 56.1 56.2 56.3 56.4 Bellerose J, Neugnot-Cerioli M, Bédard K, et al. A Highly Diverse Portrait : Heterogeneity of Neuropsychological Profiles in cblC Defect. JIMD Rep. 2016;29:19-32.
- ↑ Ricci D, Martinelli D, Ferrantini G, et al. Early neurodevelopmental characterization in children with cobalamin C/defect. J Inherit Metab Dis. 2020;43(2):367-374.
- ↑ 58.0 58.1 Sharma AP, Greenberg CR, Prasad AN, Prasad C. Hemolytic uremic syndrome (HUS) secondary to cobalamin C (cblC) disorder. Pediatr Nephrol. 2007;22(12):2097-2103.
- ↑ 59.0 59.1 59.2 Beck BB, van Spronsen F, Diepstra A, Berger RMF, Kömhoff M. Renal thrombotic microangiopathy in patients with cblC defect : review of an under-recognized entity. Pediatr Nephrol. Published online 2016:1-9.
- ↑ 60.0 60.1 Tocan V, Ohkubo K, Higashi K, et al. Reappraising newborn screening for cobalamin C disorder. Pediatr Neonatol. 2018;59(4):415-417.
- ↑ Russo P, Doyon J, Sonsino E, Ogier H, Saudubray JM. A congenital anomaly of vitamin B12 metabolism: A study of three cases. Hum Pathol. 1992;23(5):504-512.
- ↑ Brandstetter Y, Weinhouse E, Splaingard ML, Tang TT. Cor pulmonale as a complication of methylmalonic acidemia and homocystinuria (Cbl-C type). Am J Med Genet. 1990;36(2):167-171.
- ↑ Ellaway C, Christodoulou J, Kamath R, Carpenter K, Wilcken B. The association of protein-losing enteropathy with cobalamin C defect. J Inherit Metab Dis. 1998;21(1):17-22.
- ↑ 64.0 64.1 64.2 Liu Y, Ji Y, Wang Y, et al. Clinical analysis of late-onset methylmalonic acidaemia and homocystinuria, cblC type with a neuropsychiatric presentation. J Neurol Neurosurg Psychiatry. 2015;86(4):472-475.
- ↑ 65.0 65.1 65.2 Wang X, Sun W, Yang Y, Jia J, Li C. A clinical and gene analysis of late-onset combined methylmalonic aciduria and homocystinuria, cblC type, in China. J Neurol Sci. 2012;318(1-2):155-159.
- ↑ Gunduz M, Unal O, Taskin BD, Karalok ZS. Cobalamin C Deficiency: Case Report of Two Different Clinical Presentations. J Neurol Exp Neurosci. 2017;2(2):40-44.
- ↑ 67.0 67.1 67.2 Whitaker AM, Thomas NH, Krivitzky LS, Ficicioglu CH. Neuropsychological implications of Cobalamin C (CblC) disease in Hispanic children detected through newborn screening. Appl Neuropsychol Child. 2018;7(2):143-149.
- ↑ 68.0 68.1 Roze E, Gervais D, Demeret S, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60(10):1457-1462.
- ↑ 69.0 69.1 69.2 69.3 Ben-Omran TI, Wong H, Blaser S, Feigenbaum A. Late-Onset Cobalamin-C Disorder: A Challenging Diagnosis. Am J Med Genet A. 2007;143A(9):979-984.
- ↑ 70.0 70.1 70.2 70.3 70.4 Wang S Jun, Yan C Zhu, Liu Y Ming, Zhao Y Ying. Late-onset cobalamin C deficiency Chinese sibling patients with neuropsychiatric presentations. Metab Brain Dis. 2018;33(3):829-835.
- ↑ 71.0 71.1 Wu L-Y, An H, Liu J, et al. Manic-depressive Psychosis as the Initial Symptom in Adult Siblings with Late-onset Combined Methylmalonic Aciduria and Homocystinemia, Cobalamin C Type. Chin Med J (Engl). 2017;130(4):492-494.
- ↑ Brunelli SM, Meyers KEC, Guttenberg M, Kaplan P, Kaplan BS. Cobalamin C deficiency complicated by an atypical glomerulopathy. Pediatr Nephrol. 2002;17(10):800-803.
- ↑ Van Hove JLKK, Van Damme-Lombaerts R, Grünewald S, et al. Cobalamin disorder Cbl-C presenting with late-onset thrombotic microangiopathy. Am J Med Genet. 2002;111(2):195-201.
- ↑ Komhoff M, Roofthooft MT, Westra D, et al. Combined Pulmonary Hypertension and Renal Thrombotic Microangiopathy in Cobalamin C Deficiency. Pediatrics. 2013;132(2):e540-e544.
- ↑ 75.0 75.1 Cornec-Le Gall E, Delmas Y, De Parscau L, et al. Adult-onset eculizumab-resistant hemolytic uremic syndrome associated with cobalamin C deficiency. Am J Kidney Dis. 2014;63(1):119-123.
- ↑ Grangé S, Bekri S, Artaud-Macari E, et al. Adult-onset renal thrombotic microangiopathy and pulmonary arterial hypertension in cobalamin C deficiency. Lancet. 2015;386(9997):1011-1012.
- ↑ 77.0 77.1 Petropoulos TE, Ramirez ME, Granton J, et al. Renal thrombotic microangiopathy and pulmonary arterial hypertension in a patient with late-onset cobalamin C deficiency. Clin Kidney J. 2018;11(3):310-314.
- ↑ Liu J, Peng Y, Zhou N, et al. Combined methylmalonic acidemia and homocysteinemia presenting predominantly with late-onset diffuse lung disease: a case series of four patients. Orphanet J Rare Dis. 2017;12(1):1-9.
- ↑ 79.0 79.1 Navarro D, Azevedo A, Sequeira S, et al. Atypical adult-onset methylmalonic acidemia and homocystinuria presenting as hemolytic uremic syndrome. CEN Case Reports. 2018;0(0):0.
- ↑ 80.0 80.1 80.2 80.3 80.4 80.5 80.6 Aleman TS, Brodie F, Garvin C, et al. Retinal Structure in Cobalamin C Disease: Mechanistic and Therapeutic Implications. Ophthalmic Genet. 2015;36(4):339-348.
- ↑ 81.00 81.01 81.02 81.03 81.04 81.05 81.06 81.07 81.08 81.09 81.10 81.11 81.12 81.13 81.14 81.15 81.16 81.17 81.18 Huemer M, Diodato D, Schwahn B, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. 2017;40(1):21-48.
- ↑ 82.0 82.1 Collison FT, Xie YA, Gambin T, et al. Whole Exome Sequencing Identifies an Adult-Onset Case of Methylmalonic Aciduria and Homocystinuria Type C (cblC) with Non-Syndromic Bulls Eye Maculopathy. Ophthalmic Genet. 2015;36(3):270-275.
- ↑ 83.0 83.1 83.2 83.3 83.4 83.5 83.6 Gerth C, Morel CF, Feigenbaum A, Levin A V. Ocular phenotype in patients with methylmalonic aciduria and homocystinuria, cobalamin C type. J AAPOS. 2008;12(6):591-596.
- ↑ 84.00 84.01 84.02 84.03 84.04 84.05 84.06 84.07 84.08 84.09 84.10 84.11 84.12 84.13 84.14 84.15 Bonafede L, Ficicioglu CH, Serrano L, et al. Cobalamin C deficiency shows a rapidly progressing maculopathy with severe photoreceptor and ganglion cell loss. Investig Ophthalmol Vis Sci. 2015;56(13):7875-7887.
- ↑ 85.0 85.1 85.2 Mitchell GA, Watkins D, Melançon SB, et al. Clinical heterogeneity in cobalamin C variant of combined homocystinuria and methylmalonic aciduria. J Pediatr. 1986;108(3):410-415.
- ↑ 86.0 86.1 86.2 86.3 86.4 Patton N, Beatty S, Lloyd ICC, Wraith JEE. Optic atrophy in association with cobalamin C (cblC) disease. Ophthalmic Genet. 2003;21(3):151-154.
- ↑ 87.0 87.1 Ricci D, Pane M, Deodato F, et al. Assessment of Visual Function in Children with Methylmalonic Aciduria and Homocystinuria. Neuropediatrics. 2005;36(3):181-185.
- ↑ 88.0 88.1 88.2 88.3 88.4 88.5 88.6 88.7 88.8 Fuchs LR, Robert M, Ingster-Moati I, et al. Ocular manifestations of cobalamin C type methylmalonic aciduria with homocystinuria. J AAPOS. 2012;16(4):370-375.
- ↑ 89.0 89.1 89.2 89.3 89.4 Gaillard MC, Matthieu JM, Borruat FX. Retinal dysfunction in combined methylmalonic aciduria and homocystinuria (Cblc) disease: A spectrum of disorders. Klin Monbl Augenheilkd. 2008;225(5):491-494.
- ↑ 90.0 90.1 90.2 90.3 Traboulsi EI, Silva JC, Geraghty MT, Maumenee IH, Valle D, Green WR. Ocular histopathologic characteristics of cobalamin C type vitamin B12 defect with methylmalonic aciduria and homocystinuria. Am J Ophthalmol. 1992;113(3):269-280.
- ↑ 91.0 91.1 91.2 91.3 91.4 91.5 Huemer M, Simma B, Fowler B, Suormala T, Bodamer OA, Sass JO. Prenatal and postnatal treatment in cobalamin C defect. J Pediatr. 2005;147(4):469-472.
- ↑ 92.0 92.1 92.2 92.3 Trefz FK, Scheible D, Frauendienst-Egger G, et al. Successful intrauterine treatment of a patient with cobalamin C defect. Mol Genet Metab Reports. 2016;6:55-59.
- ↑ Fowler B, Leonard J V., Baumgartner MR. Causes of and diagnostic approach to methylmalonic acidurias. J Inherit Metab Dis. 2008;31(3):350-360.
- ↑ 94.0 94.1 94.2 Huemer M, Ribes A, Pasquini E, et al. Newborn screening for homocystinurias and methylation disorders: systematic review and proposed guidelines. J Inherit Metab Dis. 2015;38(6):1007-1019.
- ↑ Wilcken B, Wiley V, Hammond J, Carpenter K. Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry. N Engl J Med. 2003;348(23):2304-2312.
- ↑ Chace DH, DiPerna JC, Kalas TA, Johnson RW, Naylor EW. Rapid diagnosis of methylmalonic and propionic acidemias: quantitative tandem mass spectrometric analysis of propionylcarnitine in filter-paper blood specimens obtained from newborns. Clin Chem. 2001;47(11):2040-2044.
- ↑ McHugh DMS, Cameron CA, Abdenur JE, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: A worldwide collaborative project. Genet Med. 2011;13(3):230-254.
- ↑ 98.0 98.1 Ahrens-Nicklas RC, Serdaroglu E, Muraresku C, Ficicioglu C. Cobalamin C Disease Missed by Newborn Screening in a Patient with Low Carnitine Level. In: JIMD Reports. Vol 23. ; 2015:71-75.
- ↑ Malvagia S, Haynes CA, Grisotto L, et al. Heptadecanoylcarnitine (C17) a novel candidate biomarker for newborn screening of propionic and methylmalonic acidemias. Clin Chim Acta. 2015;450(4):342-348.
- ↑ Estrella J, Wilcken B, Carpenter K, Bhattacharya K, Tchan M, Wiley V. Expanded newborn screening in New South Wales: missed cases. J Inherit Metab Dis. 2014;37(6):881-887.
- ↑ 101.0 101.1 Morel CF, Watkins D, Scott P, Rinaldo P, Rosenblatt DS. Prenatal diagnosis for methylmalonic acidemia and inborn errors of vitamin B12 metabolism and transport. Mol Genet Metab. 2005;86(1-2):160-171.
- ↑ Zong Y, Liu N, Zhao Z, Kong X. Prenatal diagnosis using genetic sequencing and identification of a novel mutation in MMACHC. BMC Med Genet. 2015;16(1):1-6.
- ↑ Han L, Chen C, Guo F, et al. Noninvasive prenatal diagnosis of cobalamin C (cblC) deficiency through target region sequencing of cell-free DNA in maternal plasma. Prenat Diagn. 2020;40(3):324-332.
- ↑ 104.0 104.1 104.2 104.3 104.4 Bartholomew DW, Batshaw ML, Allen RH, et al. Therapeutic approaches to cobalamin-C methylmalonic acidemia and homocystinuria. J Pediatr. 2006;112(1):32-39.
- ↑ 105.0 105.1 Ogier de Baulny H, Gérard M, Saudubray JM, Zittoun J. Remethylation defects: guidelines for clinical diagnosis and treatment. Eur J Pediatr. 1998;157(S2):S77-S83.
- ↑ Andersson HC, Shapira E, H.C. A, E. S. Biochemical and clinical response to hydroxocobalamin versus cyanocobalamin treatment in patients with methylmalonic acidemia and homocystinuria (cblC). J Pediatr. 1998;132(1):121-124.
- ↑ Froese DS, Zhang J, Healy S, Gravel RA. Mechanism of vitamin B12-responsiveness in cblC methylmalonic aciduria with homocystinuria. Mol Genet Metab. 2009;98(4):338-343.
- ↑ Chang JT, Chen YY, Liu TT, Liu MY, Chiu PC. Combined methylmalonic aciduria and homocystinuria cblc type of a Taiwanese infant with c.609G>A and c.567dupT mutations in the MMACHC gene. Pediatr Neonatol. 2011;52(4):223-226.
- ↑ 109.0 109.1 Carrillo-Carrasco N, Sloan J, Valle D, Hamosh A, Venditti CP. Hydroxocobalamin dose escalation improves metabolic control in cblC. J Inherit Metab Dis. 2009;32(6):728-731.
- ↑ 110.0 110.1 Matos IV, Castejón E, Meavilla S, et al. Clinical and biochemical outcome after hydroxocobalamin dose escalation in a series of patients with cobalamin C deficiency. Mol Genet Metab. 2013;109(4):360-365.
- ↑ 111.0 111.1 Dionisi-Vici C, Martinelli D, Ceravolo F, Boenzi S, Pastore A. Optimizing the dose of hydroxocobalamin in cobalamin C (cblC) defect. Mol Genet Metab. 2013;109(4):329-330.
- ↑ 112.0 112.1 Augoustides-Savvopoulou P, Mylonas I, Sewell AC, Rosenblatt DS. Reversible dementia in an adolescent with cblC disease: Clinical heterogeneity within the same family. J Inherit Metab Dis. 1999;22(6):756-758.
- ↑ 113.0 113.1 113.2 113.3 Schiff M, Blom HJ. Treatment of inherited homocystinurias. Neuropediatrics. 2012;43(6):295-304. doi:10.1055/s-0032-1329883
- ↑ Brunel-Guitton C, Costa T, Mitchell GA, Lambert M. Treatment of cobalamin C (cblC) deficiency during pregnancy. J Inherit Metab Dis. 2010;33(SUPPL. 3):1-4.
- ↑ Manoli I, Myles JG, Sloan JL, Shchelochkov OA, Venditti CP. A critical reappraisal of dietary practices in methylmalonic acidemia raises concerns about the safety of medical foods. Part 1: isolated methylmalonic acidemias. Genet Med. 2016;18(4):386-395.