Varicella Zoster Virus Stromal Keratitis and Endotheliitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Herpes zoster ophthalmicus (HZO) describes ocular complications as a result of reactivation of the varicella- zoster virus (VZV) involving the trigeminal nerve. Corneal manifestations in HZO includes stromal keratitis and endotheliitis.
Disease
Infection by varicella zoster virus (VZV) can affect nearly every ocular tissue, including the corneal epithelium, stroma, and endothelium. The typical pathway for corneal involvement is through the reactivation of latent VZV causing herpes zoster ophthalmicus (HZO). VZV stromal keratitis (VZV-SK) is characterized by stromal inflammation and can lead to neovascularization and scarring. VZV endotheliitis (VZV-E) is characterized by keratic precipitates with overlying edema.[1] Corneal involvement is one of the leading risk factors for HZO-related visual morbidity, and if untreated can lead to permanent vision loss. Therefore, prompt identification and treatment of VZV-SK and VZV-E is important for improving clinical outcomes.
Etiology
VZV is one of eight herpes viruses that infect humans. As an alpha herpesvirus, it has a short virus life cycle and the ability to establish permanent latency, primarily in the sensory neurons of dorsal root ganglia via retrograde transport or T-cell mediated transport. Whereas herpes simplex virus latency is typically confined to cranial or sacral ganglia, VZV can establish latency along the entire neuraxis.[2] VZV can reactivate due to various stresses, including fever, surgery, trauma, and most notably, immunosuppression.[3][4] Reactivated VZV, classified as herpes zoster, replicates and travels anterograde along the sensory neural pathway, causing neuronal cell damage, demyelination, and a dermatomal vesicular rash. Primary infection with varicella can cause keratitis, but this is rare.[5] Common dermatomes affected are located in the thoracic and lumbar region, spanning T3 to L2. The ophthalmic (V1) division of the trigeminal nerve is the most frequently affected individual dermatome.[2] Because ocular tissues such as the cornea, eyelids, and conjunctiva are innervated by the nasociliary branch of V1, herpes zoster infection can lead to serious ocular complications, including stromal keratitis and endotheliitis.
Epidemiology
Studies indicate that 95 – 99% of the US adult population is seropositive for VZV.[6][7] Herpes zoster incidence ranges from 1.2 - 3.4 per 1000 people each year among young individuals, whereas incidence increases to 10 - 14 per 1000 people per year for those over 65 years of age.[8] An estimated 1 in 3 people will develop herpes zoster in their lifetime. Several studies have found that the age- and sex-adjusted incidence of herpes zoster is increasing.[9][10][11] Not only is the incidence growing, but there is also evidence suggesting that eye involvement has increased by 23% each decade from 1980 to 2007.[11]
Over 1 million cases of herpes zoster are reported per year, with 10-20% of cases affecting ocular tissue due to involvement of the V1 branch of the trigeminal nerve, gaining categorization as HZO.[6] HZO manifests commonly as conjunctivitis, keratitis, or uveitis; however, the proportions vary widely across studies.[11][12][13][14][15] For VZV-SK and VZV-E specifically, 6-16% of HZO patients with ocular manifestations have stromal keratitis, whereas kerato-uveitis/endotheliitis is less frequent at 1-7%.[13] Several studies indicate that the mean age of HZO onset is decreasing, possibly reflecting a weaker life-long immunity acquired from varicella vaccination in contrast to natural infection.[16][17] Treated HZO can remain chronic in up to 30% of patients and up to 70% of patients over 80 years of age despite antiviral treatment.[18]
Clinical Manifestations
In diagnosing HZO, one of the most common signs is a cluster of vesicular lesions developing along a V1 distribution (i.e. the frontal and nasociliary branches). Lesions on the side or tip of nose, known as Hutchinson’s sign, are an indicator of nasociliary branch involvement; however, it is crucial to note that not all HZO cases involve Hutchinson’s sign. VZV-SK and VZV-E often present within one month of dermatitis, and are typically preceded by development of punctate epitheliopathy and formation of pseudodendrites within 10 days.[15]
Varicella Zoster Virus Stromal Keratitis
Earliest involvement of the corneal stroma typically occurs after about 10 days, and depending on the region affected can be divided into anterior stromal keratitis and deep stromal keratitis. Anterior stromal keratitis is often observed first during the 2nd week of disease and is often accompanied by nummular infiltrates in the stroma just below the epithelium – these infiltrates are considered to be an immune-mediated response to viral antigens.[15] Without proper treatment, chronic stromal inflammation may result in neovascularization and/or corneal scarring. While uncommon, deep stromal keratitis can manifest several months later.[15]
Varicella Zoster Virus Endotheliitis
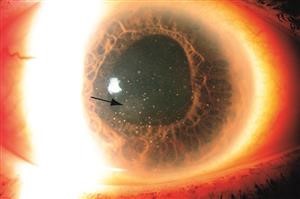
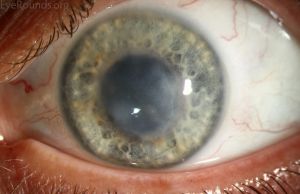
Endotheliitis presents clinically with corneal edema, keratic precipitates, and mild anterior chamber inflammation, and can lead to significant loss of endothelial cells.[19] Often, it coexists with uveitis.[20] Chronologically, Descemet folds often appear between 4 – 7 days.[15] According to Liesegang, corneal endotheliitis can be classified into four forms: linear, sectorial, disciform (Figure 2), and diffuse (Figure 1), depending on the arrangement of keratic precipitates and accompanying stromal/epithelial edema.[21] These forms have been previously reported with VZV-E.[22][23][24][25][26] Inflammation also can lead to chronic edema, endothelial decompensation, and an associated increase in intraocular pressure.
Pathophysiology
Due to various reasons, it is difficult to study herpes zoster pathophysiology compared to its relative, herpes simplex virus. Damage to the corneal stroma in VZV-SK has long been thought to result from inflammatory processes, rather than direct viral cytolytic activity. Nevertheless, evidence has shown both the presence of VZV DNA in the stroma, as well as viral capsids in keratocytes, which maintains the possibility of direct infection and long-term viral replication in the cornea.[27] Indeed, VZV DNA has been found up to 10 years after HZO in the corneal stroma.[28][29] Como, et al. recently demonstrated that VZV infection of human keratocytes resulted in widespread cell death and downregulation of inflammatory pathways.[30] In recurrent keratitis, it is unclear whether the cornea can serve as a reservoir for future episodes, or if VZV otherwise reactivates more proximal to the sensory ganglion.
There is less evidence of direct VZV invasion of endothelial cells leading to endotheliitis. One case report does describe finding viral capsid in endothelial cells, and VZV DNA has been detected in nearby arteries and perivascular regions up to 10 years post-infection.[29][31] Based on chronology of symptoms in one case, Reijo, et al. suggested that direct endothelial viral invasion is possible, as endothelial damage seemed to occur before anterior chamber flare and keratic precipitate formation.[19]
In summary, while the tissue damage and scarring seen in varicella zoster virus stromal keratitis and endotheliitis are thought to be secondary to the host’s own immune response to the virus, there is evidence that the virus may directly infect keratocytes and endothelial cells, and that it may persist in the cornea and surrounding tissues for an extended duration.
Diagnosis
The unique presentation of HZO, in which there is a unilateral painful vesicular rash in a dermatomal distribution, is typically sufficient to make the diagnosis, especially in an immunocompetent individual. Unilateral pain or hyperesthesia in the affected eye, forehead, and top of the head may present as a prodrome. An atypical presentation warrants the evaluation of other infectious and non-infectious causes. Herpes simplex, impetigo, preseptal cellulitis, contact dermatitis, and atopic eczema are etiologies that should be considered.
Furthermore, viral DNA can be detected in skin lesions or the tear film by polymerase chain reaction.[32] Corneal scrapings can be used for Tzanck smear, however, the results will not differentiate between varicella zoster and herpes simplex. Cultures can be used for immunofluorescence assays to look for IgM specific to VZV.[33] Because the majority of adults have had primary varicella infection, they will have antibodies against varicella zoster virus, and thus serology is rarely helpful in diagnosis.[32]
Management
Medical Therapy
The standard approach in treating HZO includes oral antiviral therapy such as acyclovir, valacyclovir, or famciclovir with the prospect of limiting VZV replication. Antiviral therapy started within 72 hours from symptom onset can reduce the duration of viral shedding, promote resolution of skin lesions, and limit the duration of pain.[34]
In a randomized, double-blinded, multicenter study, the safety and efficacy of oral valacyclovir 1,000 mg three times daily for 7 or 14 days was compared to oral acyclovir 800 mg five times daily for 7 days in immunocompetent adults with herpes zoster. In addition to simpler dosing (three times per day) with valacyclovir, it accelerated the resolution of pain and neuritis compared to acyclovir.[35] The ongoing Zoster Eye Disease Study aims to evaluate whether prolonged suppressive treatment with 1 g valacyclovir daily for 12 months reduces rates of new or worsening keratitis (dendritiform epithelial keratitis, stromal keratitis, and endotheliitis) or iritis, as well as the severity of post-herpetic neuralgia.[36]
While oral antiviral therapy is preferred in immunocompetent individuals, intravenous acyclovir, in the dose of 10 mg/kg three times daily for seven days, should be considered if the patient is immunocompromised and/or if there is multi-dermatome involvement.[37]
In the case of stromal keratitis or endotheliitis, topical corticosteroids should be initiated.[38] With the initiation of topical corticosteroids, recurrent inflammation can be minimized by reducing corticosteroids gradually, but it is important to educate patients that a low dose steroid may be needed indefinitely.[39] This chronic treatment increases the likelihood of cataract and steroid-induced glaucoma.
Long term complications, such as dry eye and meibomian gland dysfunction, should be addressed to decrease the risk of further damage. The use of ocular lubrication, punctal occlusion, a bandage contact lens, and more invasive procedures such as tarsorrhaphy, eyelid reconstructive surgery, and conjunctival flaps can be used in treating complications arising from VZV-SK and VZV-E.[39] Scleral contact lenses have also been shown to improve irregular astigmatism in cases of corneal scarring, as well as ocular surface rehabilitation in patients with neurotrophic keratopathy and persistent epithelial defects.[40]
Surgical Therapy
Patients with substantial corneal scarring not correctable by rigid/scleral contact lenses may be candidates for surgical treatment. Careful patient selection is critical to successful surgery in patients with VZV-SK. Phototherapeutic keratectomy (PTK) or photorefractive keratectomy (PRK) should only be considered in patients with a healthy ocular surface and normal corneal sensation. With regards to corneal transplantation, surgeons may consider deep anterior lamellar keratoplasty (DALK) and penetrating keratoplasty (PKP). Longer quiescent periods (mean periods ranging from 85 to 112 months) are associated with increased graft success and long-term graft clarity.[41] Concurrent lateral tarsorraphy should be considered in patients with a history of severe dry eye, persistent epithelial defect, neurotrophic keratopathy, exposure keratopathy, previous graft failure, history of other infectious keratitis, and history of corneal melt. Ideally, pre-existing ocular surface disease should be optimized prior to surgery. Preoperative treatments for corneal neovascularization such as argon laser, cryotherapy, or fine-needle diathermy should also be considered; it is unclear if these improve graft success.[40] Amniotic membrane transplantation (AMT) may be considered for treating persistent epithelial defects and ulceration that are refractory to conventional treatment.[42]
There is evidence that VZV-E can develop following keratoplasty for corneal decompensation. One study identified two cases of VZV-E that occurred after a penetrating keratoplasty as well as a Descemet stripping automated endothelial keratoplasty (DSAEK).[23] In both instances, a diffuse pattern of keratic precipitates was identified.
Prophylactic Therapy
The live-attenuated varicella zoster vaccine is routinely administered to children from the age of 12 months and then another dose between four and six years. Two doses of Shingrix, the recombinant zoster vaccine, should be separated by 2 - 6 months for immunocompetent adults aged 50 years and older. Both vaccines boost cell-mediated immunity against VZV, which then reduces the risk of reactivation.[32] An observational study using the live-attenuated vaccine in patients over 60 years reported that it reduced the risk of eye involvement by almost two-thirds.[43] However, an increase in cell-mediated immunity may also lead to a recurrent stromal keratitis in response to persistent viral antigens in the cornea. A quiescent period of at least one year prior to vaccination should be strongly considered in patients with a history of VZV keratitis. Close monitoring of patients after vaccination is also recommended.[40]
References
- ↑ Suzuki T, Ohashi Y. Corneal endotheliitis. Semin Ophthalmol. 2008;23(4):235-240.
- ↑ 2.0 2.1 Gilden D, Nagel MA, Cohrs RJ, Varicella-zoster. Handb Clin Neurol. Vol 123.; 2014.
- ↑ Thomas SL, Wheeler JG, Hall AJ. Case-control study of the effect of mechanical trauma on the risk of herpes zoster. BMJ. 2004;328(7437):439.
- ↑ Häfelfinger R. Simultaneous VZV and HSV-1 Reactivation after Minor Head Injury. Eur J Case Rep Intern Med. 2020;7(12):001746.
- ↑ Matoba AY, Meghpara B, Chevez-Barrios P. Varicella-zoster virus detection in varicella-associated stromal keratitis. JAMA Ophthalmol. 2014;132(4):505-506.
- ↑ 6.0 6.1 Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):3-12.
- ↑ Reynolds MA. Varicella seroprevalence in the U.S.: data from the National Health and Nutrition Examination Survey, 1999-2004. Public Health Rep. 2010;125(6):860-869.
- ↑ Schmader K. Herpes Zoster. Clin Geriatr Med. 2016;32(3):539-553.
- ↑ Hales CM. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med. 2013;159(11):739-745.
- ↑ Kawai K. Increasing Incidence of Herpes Zoster Over a 60-year Period From a Population-based Study. Clin Infect Dis. 2016;63(2):221-226.
- ↑ 11.0 11.1 11.2 Yawn BP. Herpes zoster eye complications: rates and trends. Mayo Clin Proc. 2013;88(6):562-570.
- ↑ Tran KD. Epidemiology of Herpes Zoster Ophthalmicus: Recurrence and Chronicity. Ophthalmology. 2016;123(7):1469-1475.
- ↑ 13.0 13.1 Li JY. Herpes zoster ophthalmicus: acute keratitis. Curr Opin Ophthalmol. 2018;29(4):328-333.
- ↑ Starr CE, Pavan-Langston D. Varicella-zoster virus: mechanisms of pathogenicity and corneal disease. Ophthalmol Clin North Am. 2002;15(1):7-15,.
- ↑ 15.0 15.1 15.2 15.3 15.4 Liesegang TJ. Corneal complications from herpes zoster ophthalmicus. Ophthalmology. 1985;92(3):316-324.
- ↑ Ghaznawi N. Herpes zoster ophthalmicus: comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology. 2011;118(11):2242-2250.
- ↑ Chan AY. Factors associated with age of onset of herpes zoster ophthalmicus. Cornea. 2015;34(5):535-540.
- ↑ Johnson RW. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines. 2015;3(4):109-120.
- ↑ 19.0 19.1 Reijo A, Antti V, Jukka M. Endothelial cell loss in herpes zoster keratouveitis. Br J Ophthalmol. 1983;67(11):751-754.
- ↑ Moshirfar M. A Review of Corneal Endotheliitis and Endotheliopathy: Differential Diagnosis, Evaluation, and Treatment. Ophthalmol Ther. 2019;8(2):195-213.
- ↑ Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18(2):127-143.
- ↑ Olsen TW. Linear endotheliitis. Am J Ophthalmol. 1994;117(4):468-474.
- ↑ 23.0 23.1 Morishige N. Differential Changes in Intraocular Pressure and Corneal Manifestations in Individuals With Viral Endotheliitis After Keratoplasty. Cornea. 2016;35(5):602-606.
- ↑ Khodabande A. Varicella endotheliitis: a case report. Eur J Ophthalmol. 2009;19(6):1076-1078.
- ↑ Al Somali AI, Otaif W. Concomitant Varicella Zoster Virus and Cytomegalovirus Corneal Endotheliitis in an Immunocompetent Patient. Ocular Immunology and Inflammation. 2020;0(0):1-3. doi:10.1080/09273948.2020.1821900
- ↑ Peng R, Guo Y, Xiao G, Lu Q, Sun B, Hong J. Clinical Manifestations and Characteristics of In Vivo Confocal Microscopy in Varicella Zoster Virus-Related Corneal Endotheliitis. Ocular Immunology and Inflammation. 2019;27(8):1270-1279. doi:10.1080/09273948.2018.1521435
- ↑ Jastrzebski A. Reactivation of Herpes Zoster Keratitis With Corneal Perforation After Zoster Vaccination. Cornea. 2017;36(6):740-742.
- ↑ Wenkel H. Detection of varicella zoster virus DNA and viral antigen in human eyes after herpes zoster ophthalmicus. Ophthalmology. 1998;105(7):1323-1330.
- ↑ 29.0 29.1 Wenkel H. Detection of varicella zoster virus DNA and viral antigen in human cornea after herpes zoster ophthalmicus. Cornea. 1993;12(2):131-137.
- ↑ Como CN. Varicella Zoster Virus Induces Differential Cell-Type Specific Responses in Human Corneal Epithelial Cells and Keratocytes. Invest Ophthalmol Vis Sci. 2019;60(2):704-711.
- ↑ Maudgal PC. Varicella-zoster virus in the human corneal endothelium: a case report. Bull Soc Belge Ophtalmol. 1980;190:71-86.
- ↑ 32.0 32.1 32.2 Tuft S. How to manage herpes zoster ophthalmicus. Community eye health. 2020;33(108):71-72.
- ↑ Cason JB. Herpes Zoster Ophthalmicus. EyeWiki. Accessed May 30, 2021. https://eyewiki.aao.org/Herpes_Zoster_Ophthalmicus
- ↑ Gnann Jr. JW. Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al., eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; 2007. Accessed May 30, 2021. http://www.ncbi.nlm.nih.gov/books/NBK47401/
- ↑ Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;Jul;39(7):1546-53. doi:10.1128/aac.39.7.1546.
- ↑ NYU Langone Health. Long-Term Suppressive Valacyclovir Treatment for Herpes Zoster Ophthalmicus. clinicaltrials.gov; 2021. Accessed September 8, 2021. https://clinicaltrials.gov/ct2/show/NCT03134196
- ↑ Liesegang TJ. Diagnosis and therapy of herpes zoster ophthalmicus. Ophthalmology. Published online 1991. doi:10.1016/s0161-6420(91)32163-8.
- ↑ Cobo LM. Corneal complications of herpes zoster ophthalmicus. Prevention and treatment. Cornea. 1988;7(1):50-56.
- ↑ 39.0 39.1 Herpes Zoster Ophthalmicus. American Academy of Ophthalmology. Accessed May 30, 2021. https://www.aao.org/focalpointssnippetdetail.aspx?id=8367b620-245c-4ebf-89e7-eca0c8d35aa3
- ↑ 40.0 40.1 40.2 Hassan OM, Farooq AV, Soin K, Djalilian AR, Hou JH. Management of Corneal Scarring Secondary to Herpes Zoster Keratitis. Cornea. 2017;36(8):1018-1023. doi:10.1097/ICO.0000000000001235
- ↑ Soong HK, Schwartz AE, Meyer RF, Sugar A. Penetrating keratoplasty for corneal scarring due to herpes zoster ophthalmicus. The British journal of ophthalmology. 1989;73(1):19-21. doi:10.1136/bjo.73.1.19
- ↑ Heiligenhaus A. Management of acute ulcerative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation. British Journal of Ophthalmology. 2003;87(10):1215-1219. doi:10.1136/bjo.87.10.1215
- ↑ Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305(2):160-166. doi:10.1001/jama.2010.1983