Use of Artificial Intelligence in Community Eye Screenings
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Cloud based Artificial Intelligence (AI) has the ability to augment clinical ophthalmic care through expanded access to both providers and patients. As patient care often involves ocular imaging and other image-based diagnostic tools such as ocular coherence tomography (OCT), these programs can help quickly determine if there is potentially referable pathology. As the world’s population ages and the number of people suffering from eye conditions increases, creating new solutions to address this growing need is more and more important.[1] According to the Centers for Disease Control and Prevention (CDC), the primary causes of impaired vision are age-related eye diseases: age-related macular degeneration (AMD), cataracts, diabetic retinopathy (DR), and glaucoma. As of 2020, the CDC estimates that the number of Americans affected by AMD, cataracts, and DR are 2.95 million, 30.1 million, and 4.1 million respectively.[2] Vision-threatening disease is a significant public health concern, with an estimated annual expenditure of $51.4 billion dollars in the United States. The public health burden is also exacerbated by the lack of access to and/or utilization of eye care.[3] Studies have shown that there is often less than a 50% compliance with eye care referrals, which further exacerbates the disparities associated with ophthalmic care in the United States.[4] AI can also serve as a valuable tool in screenings in underserved populations by allowing local providers to triage eye pathologies in their patients and ensure emergent cases are referred appropriately to eye care providers.[5]
Community eye screenings serve as valuable opportunities for patients without regular access to eye care due to their location or socioeconomic status to receive a comprehensive eye wellness exam.[5] Unfortunately, these screenings are often limited by staff availability, as a certified reader and/or board-certified ophthalmologist is needed to interpret images and make recommendations. As such, patients can easily be lost to follow up and never receive the treatment they need to guard their vision. AI can help address this staffing issue, as retinal images and posterior pole scans from OCT can be swiftly and accurately interpreted by an AI program instead of a human reader. This is especially important as the 2019-2020 national ophthalmologist density in the United States is only 5.29 ophthalmologists per 100,000 people. This potential disparity is exacerbated in rural areas of the country where there are often no ophthalmologists for hundreds of miles.[6] These AI programs can provide an initial level of triage for patients with VTDs by severity for referral to an ophthalmologist. AI and deep learning programs may also surpass human readers in some cases, as shown in a study by Weber et al. where a deep learning algorithm for AMD was better than human readers at detecting early stages of the disease.[7]
Practical considerations of AI use
Several studies have investigated the various AI programs available to assess vision threatening diseases such as AMD, DR, glaucoma, and retinopathy of prematurity. These AI programs rely on a pattern recognition algorithm to identify the presence of a vision threatening disease (VTD).[8] [9] These programs vary in the VTDs they are able to detect, as well as their sensitivity and specificity.[10] Some programs, like RetmarkerDR for detection of Diabetic Retinopathy, even have the ability to track disease progression in a patient over time.[11] AI algorithms can also provide a level of objectivity to a diagnosis as they will consistently give the same output for a single input, whereas human readers (i.e. ophthalmologists) may vary in their clinical recommendations.[12]
A major barrier to AI deployment in clinics is that there is not yet one product that can detect all potential VTDs, but there are upcoming technologies that provide more broad detection. SELENA+, developed by Singaporean-based EyRIS, is an example of an AI product with the capacity to detect multiple VTDs. Currently, with the help of convolutional neural networks (CNN) and a deep learning system (DLS), SELENA+ is capable of referring diabetic retinopathy, glaucoma suspect, and age-related macular degeneration.[13] Deep learning is a type of AI that aims to imitate the structure and function of the brain in its application, and a CNN is a deep learning algorithm which assigns variable weights to various characteristics of inputted images to differentiate between them. The pipeline includes acquisition of retinal fundus photographs of the eyes, input of the photographs to a cloud-based platform, image interpretation by the neural network, and generation of a report. To use SELENA+, the only requirements for use are retinal images and internet connectivity for upload to the cloud. The uploader of the images must correctly upload the macula-centered and optic nerve-centered images to the correct fields; otherwise no special training is required to use the AI. The report can present three outcomes for each eye and disease: ungradable, referable, and non-referable. The report can subsequently be used to decide a screening participant’s next steps in terms of care. SELENA+ has been used to screen VTDs worldwide, such as in Zambia.[14]
While researchers are trying to develop programs that are capable of detecting all potential eye diseases, as an ophthalmologist may be able to do in an office appointment, the technology still possesses some limitations.[15] Concerns include some programs’ high false-negative rates of detection of retinal diseases, false-positive errors that can lead to unnecessary treatment, difficulty in evaluating the AI’s reasoning behind the output, and patients’ distrust of computer-generated diagnoses.[16] Furthermore, many of these AI programs also cannot yet assign severity to a condition. A 2020 study on a deep learning program’s ability to detect retinal hemorrhage found that it could only reliably detect the hemorrhage, not assign a specific diagnosis to it. For example, the program could not distinguish between peripheral hemorrhage or macular hemorrhage, the latter of which is an ophthalmic emergency.[17][18]
AI Integration at Rutgers New Jersey Medical School Institute of Ophthalmology and Vision Sciences
At Rutgers New Jersey Medical School (NJMS), there has been robust AI-human collaboration at the Department of Ophthalmology and Visual Sciences for several years. The COVID-19 pandemic brought discussions of telemedicine to the forefront of clinical care. This was especially evident in ophthalmology clinics where patients tend not to seek ophthalmic care for various reasons even in non-pandemic times.[19] [20]
Rutgers NJMS is located in Newark, New Jersey and provides medical services to a community in which 27.4% of people live below the federal poverty line, and 19.1% of people have no health insurance.[21] The trends in socioeconomic status are similar for the greater Newark area, and with patients often skipping eye examinations due to no perceived need, lack of education around eye health, and lack of eye insurance to see an eye care professional, there is a marked need for vision services.[3] The Student Sight Savers Program (SSSP), in collaboration with the Department of Ophthalmology at University Hospital (UH) and Rutgers NJMS, has partnered with various community organizations to meet this gap in care through performing community eye screenings. At each screening, volunteers assign each subject an anonymous ID and record demographics including age, gender, race, smoking history, and self-reported history of diabetes, glaucoma, AMD or hypertension. In addition, volunteers use various instruments, such as Snellen chart, automated puff tonometer, autorefractor, and non-mydriatic retinal fundus camera and optical coherence tomography (OCT), to collect information about each subject’s eye health (Figures 1-2). The team also uses TeamViewer, a remote access software, and a Telepresence Double-2 robot (California, USA) to share data and communicate with a specialist remotely (Figure 2). Recently, the team has also incorporated SELENA+ to help identify VTDs and indicate need for additional imaging such as OCT. An on-site certified reader then interprets the data and advises the subject on need for referral and follow-up.
Through earlier detection of disease, these screenings aim to encourage prompt initiation of lifestyle modifications and/or medical intervention, which can delay disease progression and improve outcomes in these vulnerable populations. These annual screenings provide advice from an eye care professional and continuity of care for individuals who do not have access to preventative care. With AI integration, these screenings may be able to expand through increased efficiency of analysis, which will help the program better address the gap in eye care in the greater Newark area.
Global Impact and Collaborative Initiatives
In the realm of global health, collaborative endeavors are shaping the landscape of eye care delivery, particularly in underserved regions with limited resources.[22] Partnerships between academic institutions, non-profit organizations, and technology companies are spearheading initiatives to deploy AI-powered screening programs, addressing significant disparities in access to eye care worldwide.[23] Initiatives such as the Global Initiative on AI for Health (GI-AI4H) exemplify this collaborative spirit, bringing together diverse stakeholders to develop tailored AI-driven solutions for tackling health (and eye) care disparities.[24] Through joint research projects and capacity-building workshops, this consortium equips local healthcare providers with the tools and knowledge needed to leverage AI effectively in underserved communities.
Furthermore, programs like "Vision for All" lead the charge in scaling up AI-powered screening programs across multiple countries.[25] By fostering cross-sector partnerships and sharing best practices, these initiatives aim to create sustainable solutions that can combat avoidable blindness and improve vision outcomes on a global scale.[22] Innovation and collaboration are at the forefront of these efforts, as technology companies develop customized AI algorithms and software platforms optimized for low-resource settings.[26] By harnessing the collective expertise and resources of diverse stakeholders, collaborative initiatives are driving positive change and advancing the mission of global eye health equity.
Future Direction of AI Incorporation in Ophthalmic Care
While many of these AI’s are not yet FDA-approved in the United States, they have received approval from health authorities abroad like the Singapore’s Health Science Authority (HSA), Medical Device Authority of Malaysia, and European Union Conformité Européenne (CE) Mark.[27] Currently, two ophthalmology AIs are FDA-approved for use in the United States: EyeNuk’s EyeArt and Digital Diagnostics’ IDx-DR, both of which are approved to detect DR . They are both approved for autonomous use, which means use of their diagnoses without human input.[28] [29] AI’s potential for clinical use is gaining recognition, as research has demonstrated clinically acceptable performance of AIs.[30] As the public health burden of eye disease increases, the need for screenings will also rise. AI can be a cost-effective way to meet this demand, whether in community screenings or in the clinical setting.[31] [32]
Pairing AI with other technology, such as telemedicine systems, can further expand its reach. For example, in the screenings led by Rutgers NJMS and UH, the ophthalmologist connecting via the telemedicine robot can use remote camera access software to consider the AI’s report when advising the screening participant. In addition, AI’s diagnostic potential is still reaching new heights. Current AI products demonstrate remarkable success in analyzing posterior segment images; some researchers are expanding their focus to AI’s capability in reading anterior segment images.[12] Advancements are also being made in the development of AI that may interpret a patient’s status over time, which may help determine progression or improvement of disease severity.
Figures/Images
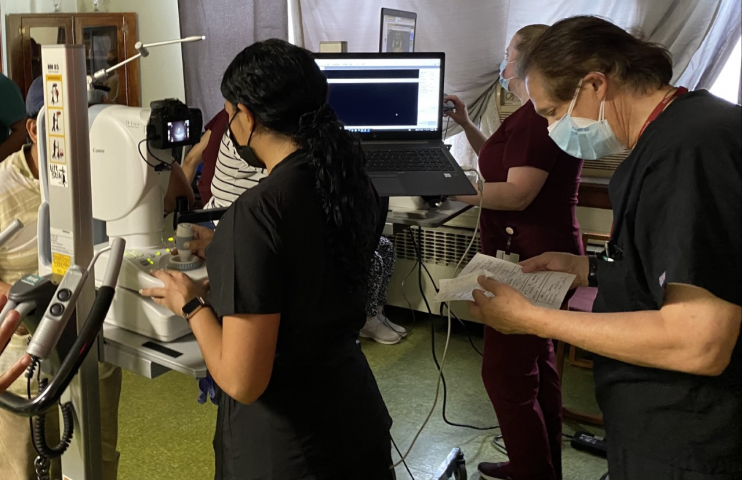
Figure 1. SSSP Volunteers using non-mydriatic retinal cameras to capture images of a patient’s anterior segment and posterior pole (retina), while certified reader Dr. Bernard Szirth interprets patient data. Note subjects as well as imagers all stand at the instruments to streamline the process. When subjects sit at an instrument, the process can take 40% longer. Especially in times of COVID-19, it is important to minimize time at each data collecting station to limit exposure.
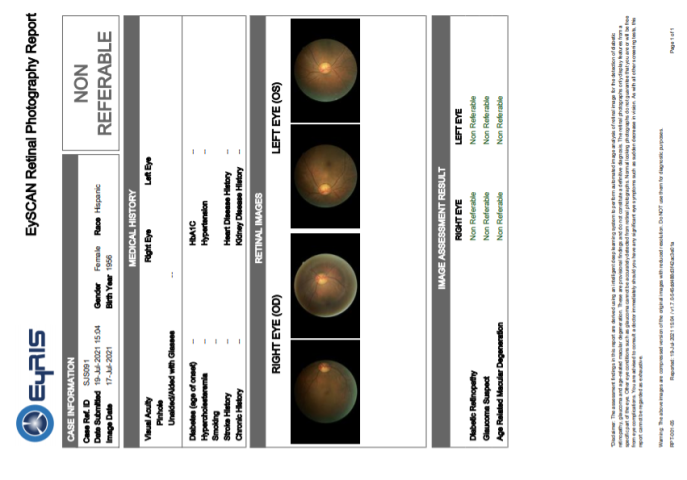
Figure 5. Example report generated by SELENA+, indicating no need for referral for diabetic retinopathy, glaucoma suspect, and age-related macular degeneration. On the report itself, retinal photographs are thumb prints set on low resolution so as to accelerate the download from the cloud to the end-user.
References
- ↑ Balyen, L., & Peto, T. (2019). Promising artificial intelligence-machine learning-deep learning algorithms in ophthalmology. The Asia-Pacific Journal of Ophthalmology, 8(3), 264-272.
- ↑ Centers for Disease Control and Prevention. (2020, June 3). Common eye disorders and diseases. Centers for Disease Control and Prevention. https://www.cdc.gov/visionhealth/basics/ced/index.html.
- ↑ Jump up to: 3.0 3.1 Elam AR, Lee PP. Barriers to and Suggestions on Improving Utilization of Eye Care in High-Risk Individuals: Focus Group Results. Int Sch Res Notices. 2014;2014:527831. Published 2014 Oct 15. doi:10.1155/2014/527831
- ↑ Brinks, M., Zaback, T., Park, D.-W., Joan, R., Cramer, S. K., & Chiang, M. F. (2018). Community-based vision health screening with on-site definitive exams: Design and outcomes. Cogent Medicine, 5(1), 1560641. https://doi.org/10.1080/2331205x.2018.1560641
- ↑ Jump up to: 5.0 5.1 Shahid, K., Kolomeyer, A. M., Nayak, N. V., Salameh, N., Pelaez, G., Khouri, A. S., Eck, T. T., & Szirth, B. (2012). Ocular telehealth screenings in an urban community. Telemedicine journal and e-health : the official journal of the American Telemedicine Association, 18(2), 95–100. https://doi.org/10.1089/tmj.2011.0067
- ↑ Area Health Resources Files. (2020). Retrieved August 27, 2021, from Hrsa.gov website: https://data.hrsa.gov/topics/health-workforce/ahrf
- ↑ Grassmann, F., Mengelkamp, J., Brandl, C., Harsch, S., Zimmermann, M. E., Linkohr, B., ... & Weber, B. H. (2018). A deep learning algorithm for prediction of age-related eye disease study severity scale for age-related macular degeneration from color fundus photography. Ophthalmology, 125(9), 1410-1420.
- ↑ Artificial Intelligence in Retina - EyeWiki. (2020). Retrieved August 1, 2021, from Eyewiki.org website: https://eyewiki.org/Artificial_Intelligence_in_Retina#cite_note-:4-1
- ↑ Artificial Intelligence in Glaucoma - EyeWiki. (2021). Retrieved August 1, 2021, from Eyewiki.org website: https://eyewiki.org/Artificial_Intelligence_in_Glaucoma#cite_note-:1-1
- ↑ Kapoor, R., Walters, S. P., & Al-Aswad, L. A. (2019). The current state of artificial intelligence in ophthalmology. Survey of ophthalmology, 64(2), 233-240.
- ↑ Leicht, S. F., Kernt, M., Neubauer, A., Wolf, A., Oliveira, C. M., Ulbig, M., & Haritoglou, C. (2014). Microaneurysm turnover in diabetic retinopathy assessed by automated RetmarkerDR image analysis-potential role as biomarker of response to ranibizumab treatment. Ophthalmologica, 231(4), 198-203.
- ↑ Jump up to: 12.0 12.1 Roach, Linda. (2017). Artificial Intelligence. Retrieved August 1, 2021, from American Academy of Ophthalmology website: https://www.aao.org/eyenet/article/artificial-intelligence
- ↑ Ting DS, Cheung CY-L, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211-2223. doi:10.1001/jama.2017.18152
- ↑ Bellemo, Valentina & Lim, Zhan & Lim, Gilbert & Nguyen, Duc Quang & Xie, Yuchen & Yip, Michelle & Hamzah, Haslina & Ho, Jinyi & Lee, Xin & Hsu, Wynne & Lee, Mong & Musonda, Lillian & Chandran, Manju & Chipalo-Mutati, Grace & Muma, Mulenga & Tan, Gavin & Sivaprasad, Sobha & Menon, Geeta & Wong, T-Y & Ting, Daniel. (2019). Artificial Intelligence using Deep Learning to Screen for Referable and Vision-threatening Diabetic Retinopathy in Africa: a Clinical Validation Study. 1. e35-44. 10.1016/S2589-7500(19)30004-4.
- ↑ Ravindran, S. (2019). How artificial intelligence is helping to prevent blindness. Nature.
- ↑ Moraru AD, Costin D, Moraru RL, Branisteanu DC. Artificial intelligence and deep learning in ophthalmology - present and future (Review). Exp Ther Med. 2020;20(4):3469-3473. doi:10.3892/etm.2020.9118
- ↑ Li, Z., Guo, C., Nie, D., Lin, D., Zhu, Y., Chen, C., ... & Lin, H. (2020). Development and evaluation of a deep learning system for screening retinal hemorrhage based on ultra-widefield fundus images. Translational vision science & technology, 9(2), 3-3.
- ↑ Lu, Z.E., Chen, Y., Ye, C., Ooms, A., Ledesma, G.., Duong, K., Szirth, B., Khouri, A.S. Accuracy of Automated Retinal Software for Diabetic Retinopathy Detection in Type I Diabetics vs Human Graders. Invest. Ophthalmol. Vis. Sci. 2020;61(7):1650.
- ↑ Zhang, I, Zhou B, Crane, A, Ye, C; Patton A, Szirth, B; Khouri, AS. Vision Threatening Disease Triage Using Tele-Ophthalmology during COVID-19 in the Emergency Department: A Pilot Study. Invest. Ophthalmol. Vis. Sci. 2021;62(8):1893.
- ↑ Zhou, B; Zhang, I; Patton, A; Ye, C; Szirth, B; Khouri, AS. Telemedicine Screening of Vision Threatening Diseases During the COVID-19 Pandemic. Invest. Ophthalmol. Vis. Sci. 2021;62(8):1899.
- ↑ QuickFacts: Newark city, New Jersey; United States. (2019). Retrieved August 17, 2021, from Census Bureau QuickFacts website: https://www.census.gov/quickfacts/fact/table/newarkcitynewjersey,US/PST045219
- ↑ Jump up to: 22.0 22.1 Zhu A, Tailor P, Verma R, Zhang I, Schott B, Ye C, Szirth B, Habiel M, Khouri AS. Implementation of deep learning artificial intelligence in vision-threatening disease screenings for an underserved community during COVID-19. J Telemed Telecare. 2023 Mar 13:1357633X231158832. doi: 10.1177/1357633X231158832. Epub ahead of print. PMID: 36908254; PMCID: PMC10014445.
- ↑ Lin S, Ma Y, Xu Y, Lu L, He J, Zhu J, Peng Y, Yu T, Congdon N, Zou H. Artificial Intelligence in Community-Based Diabetic Retinopathy Telemedicine Screening in Urban China: Cost-effectiveness and Cost-Utility Analyses With Real-world Data. JMIR Public Health Surveill. 2023 Feb 23;9:e41624. doi: 10.2196/41624. PMID: 36821353; PMCID: PMC9999255.
- ↑ Global Initiative on AI for Health, World Health Organization, https://www.who.int/initiatives/global-initiative-on-ai-for-health, accessed February 24, 2024
- ↑ Vision for All, https://www.visionforall2020.org/, accessed February 24, 2024
- ↑ Li Z, Wang L, Wu X, Jiang J, Qiang W, Xie H, Zhou H, Wu S, Shao Y, Chen W. Artificial intelligence in ophthalmology: The path to the real-world clinic. Cell Rep Med. 2023 Jul 18;4(7):101095. doi: 10.1016/j.xcrm.2023.101095. Epub 2023 Jun 28. PMID: 37385253; PMCID: PMC10394169.
- ↑ Nova Group. EYRIS Awarded 5-Year Contract to Deploy SELENA+. Nova Group.
- ↑ Ting DS, Cheung CY-L, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211-2223. doi:10.1001/jama.2017.18152
- ↑ Jennifer Lim, Malavika Bhaskaranand, Chaithanya Ramachandra, Sandeep Bhat, Kaushal Solanki, and Srinivas Sadda. “Artificial Intelligence Screening for Diabetic Retinopathy: Analysis from a Pivotal Multi-Center Prospective Clinical Trial.” In ARVO Imaging in the Eye Conference 2019. Vancouver, BC, Canada, 2019.
- ↑ Gunasekeran, D. and Wong, T., 2020. Artificial Intelligence in Ophthalmology in 2020: A Technology on the Cusp for Translation and Implementation. Asia-Pacific Journal of Ophthalmology, 9(2), pp.61-66.
- ↑ Yuchen Xie, Quang Nguyen, Valentina Bellemo, Michelle Y.T. Yip, Xin Qi Lee, Haslina Hamzah, Gilbert Lim, Wynne Hsu, Mong Li Lee, Jie Jin Wang, Ching-Yu Cheng, Eric Andrew Finkelstein, Ecosse Luc Lamoureux, Gavin Siew Wei Tan, Tien Yin Wong, Daniel SW Ting; Cost-Effectiveness Analysis of an Artificial Intelligence-Assisted Deep Learning System Implemented in the National Tele-Medicine Diabetic Retinopathy Screening in Singapore. Invest. Ophthalmol. Vis. Sci. 2019;60(9):5471.
- ↑ Lin, H., Li, R., Liu, Z., Chen, J., Yang, Y., Chen, H., Lin, Z., Lai, W., Long, E., Wu, X., Lin, D., Zhu, Y., Chen, C., Wu, D., Yu, T., Cao, Q., Li, X., Li, J., Li, W., Wang, J., … Liu, Y. (2019). Diagnostic Efficacy and Therapeutic Decision-making Capacity of an Artificial Intelligence Platform for Childhood Cataracts in Eye Clinics: A Multicentre Randomized Controlled Trial. EClinicalMedicine, 9, 52–59. https://doi.org/10.1016/j.eclinm.2019.03.001