Artificial Intelligence in Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Glaucoma is a disease which is still not completely understood, even though it is the most common cause of irreversible blindness. There is still a lot of unknowns in the area of glaucoma suspects. In addition to intraocular pressure (IOP), optic disc changes, retinal nerve fiber layer (RNFL) defects, ganglion cell layer (GCL) defects, gonioscopy, and visual fields are important diagnostic criteria with no definitive cut-offs. Family history, systemic diseases, and social determinants of health also influence the risk of glaucoma. This is a lot of information which has to be looked at holistically to arrive at a diagnosis.
Artificial intelligence (AI) and machine learning (ML) will play a major role in ophthalmology[1] as they are particularly suited to extract useful information from a lot of data. With the help of AI, we might finally be able to diagnose glaucoma in borderline cases and start appropriate treatment early. We may even be able to predict the course of glaucoma.
Introduction
Artificial Intelligence has slowly been incorporated in several of the gadgets and software that we use daily. It seems inevitable that AI will become a routine part of our medical practice. Ophthalmology is particularly suited to the application of AI as there is a lot of clinical imaging, scans, and tests that need interpretation.[2]
Glaucoma in particular has a huge potential for AI[3] related advances as data from multiple modalities has to be combined together in a meaningful way to arrive at a management plan. If the patient prognosis with different management protocols can be estimated by a predictive algorithm, it would greatly help in guiding the appropriate treatment and improving adherence to treatment.
Artificial Intelligence in Ophthalmology[1]
The role of AI in Ophthalmology is potentially vast as there are a lot of data that can be processed to yield useful information. There have been multiple programming competitions and hackathons where several teams compete to develop image processing algorithms to detect diabetic retinopathy and glaucoma.
The ophthalmic diseases where AI is being used are diabetic retinopathy, glaucoma, age-related macular degeneration, retinopathy of prematurity, retinal vascular occlusions, keratoconus, cataract, refractive errors, retinal detachment, strabismus, and ocular cancers.[1] It is also useful for intraocular lens power calculation, planning strabismus surgeries, and planning intravitreal antivascular endothelial growth factor injections. In addition, AI can detect cognitive impairment, dementia, Alzheimer's disease, stroke risk, and so on from fundus photographs and optical coherence tomography.
Basic Definitions
Artificial Intelligence (AI)
Defined as "theory and development of computer systems able to perform tasks normally requiring human intelligence, such as visual perception, speech recognition, decision-making, and translation between languages."[4] Artificial Intelligence is the application of computer algorithms to replicate intelligent human-like behavior.
Machine Learning (ML)
Defined as "the capacity of a computer to learn from experience, i.e. to modify its processing on the basis of newly acquired information."[5] In Machine Learning, the algorithms learn on their own by trial and error, without being explicitly programmed the steps to do a task.
Neural Network (NN)
Defined as "a computer system modeled on the human brain and nervous system."[6] The software is built as a network of neurons communicating with each other with multiple inputs modifying the output, and often have a feedback loop for learning. Deep learning is a sub-type of neural networks. Convolutional neural networks (CNN) are one type of neural network layer which is commonly found in image processing. Generative adversarial networks (GAN) is a different class of neural network (often itself implemented as a deep learning neural network.)
Deep Learning (DL)
Deep learning is a class of machine learning algorithms whose neural network architecture uses many layers to progressively extract higher level features from the raw input. For example, in image processing, lower layers may identify edges, while higher layers may identify the concepts relevant to a human such as digits or letters or faces.[7] It is an advanced level of neural networks. Individual layers in the DL architecture may use convolutional neural networks (CNN).
Artificial Intelligence in Glaucoma[8][9]
Optic disc photographs, visual fields, retinal nerve fiber layer thickness, ganglion cell layer thickness, intraocular pressure, and gonioscopy all have to be carefully evaluated and monitored for a proper glaucoma workup. Artificial Intelligence is well-suited to extract useful information from all this data. Such a comprehensive glaucoma AI is not yet ready, but would indeed be a game-changer in the fight against this "silent thief of sight".
Meanwhile, there has been tremendous progress in the use of AI for evaluation of individual parameters and some combinations. There has been success in the diagnostic as well as prognostics aspects. A comprehensive AI for glaucoma should evaluate all the parameters including IOP, disc morphology, gonioscopy, visual fields, and OCT together.
Intraocular Pressure (IOP)
Prediction of IOP trends from previous data and medications would be a useful and plausible use of AI. As of now, AI was used for evaluation of data from Sensimed Triggerfish (Sensimed AG, Lausanne, Switzerland), which is a contact lens based continuous IOP monitoring device which actually measures only the corneal strain changes due to IOP fluctuations. Martin et al used data from 24 prospective studies of Triggerfish using random forests (a machine learning method) to identify the parameters associated with glaucoma patients.[10]
Optic Disc Photography
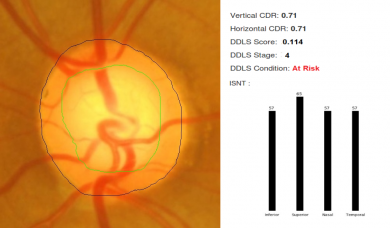
For fundus photographs, Li et al evaluated a deep learning algorithm that showed a high sensitivity (95.6%) and specificity (92%) to detect referable glaucomatous optic neuropathy.[11] The disadvantage was that high myopia caused false negatives and physiological cupping caused false positives. Al-Aswad et al evaluated Pegasus (Visulytix Ltd., London UK), a deep learning system to detect glaucomatous optic neuropathy from color fundus photographs and showed that it outperformed 5 out of 6 ophthalmologists in the study.[12] Pegasus is the AI system that is available free for use in the Orbis Cybersight Consult Platform. NetraAI (Leben Care Technologies Pte Ltd) is another AI that evaluates glaucomatous fundus photographs. Cerentini et al used the GoogLeNet deep learning network[13] to develop an automatic classification method to detect glaucoma in fundus images. Haleem et al used a novel technique for automatic boundary detection of optic disc and cup to aid automatic glaucoma diagnosis from fundus photos.[14] Thompson and team was able to use deep learning to measure NRR loss from optic disc photos.[15] Indian startup Kalpah Innovations (Vishakapatnam, India) released Retinal Image Analysis – Glaucoma (RIA-G),[16] cloud based software in 2016 to analyse fundus images to look for the likelihood of glaucoma. It uses advanced image processing algorithms to measure disc size, cup size, C/D ratio, neuroretinal rim thickness, disc damage likelihood score (DDLS), and to look for violation of ISNT rule. It also uses deep learning to learn from the user in case of errors in automated detection.
Optical Coherence Tomography (OCT) - RNFL, GCL
OCT machines automatically measure disc size, cupping, neuroretinal rim area, RNFL thickness, and GCL thickness. All of these parameters can be estimated using AI-based image segmentation techniques.[17] Omodoka et al, using a neural network classified images from swept-source OCT into Nicolela's 4 optic disc types with high accuracy.[18] Muhammad et al showed that a hybrid deep learning method on a single wide field swept-source OCT had 93.1% sensitivity in detecting glaucoma suspects.[19] Asaoka et al evaluated a deep learning algorithm that diagnosed glaucoma based on macular OCT for RNFL and GCL.[20] Other studies by Christopher et al,[21] Barella et al,[22] Bizios et al,[23] & Larossa et al[24] evaluated unsupervised machine learning, machine learning classifiers, artificial neural networks, support vector machines, and segmentation methods for glaucoma OCT.
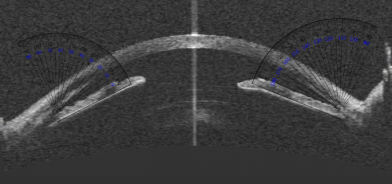
Anterior Segment OCT (AS-OCT)
AS-OCT images can also be processed for structure segmentation, measurement, and screening for angle closure using algorithms being developed.[25] Niwas et al evaluated a fully automated model to classify angle closure glaucoma from AS-OCT scans and showed an accuracy of 89.2%.[26]
Visual Fields (VF)
AI can potentially help in rapid and accurate interpretation of visual fields. Asaoka et al used a feed-forward neural network to identify pre-perimetric visual fields which did not meet Anderson-Patella's criteria.[27] Li et al evaluated a Convolutional Neural Network (CNN) to automatically differentiate glaucomatous VFs from non-glaucomatous VFs.[28] Goldbaum et al used unsupervised machine learning and variational Bayesian independent component analysis mixture model (vB-ICA-mm) to analyze VF defects.[29] Andersson, Heijl, and colleagues showed that a trained artificial neural network obtained 93% sensitivity and 91% specificity in evaluating glaucoma VF and performed at least as well as clinicians.[30] Bowd, Weinreb, and colleagues successfully used variational Bayesian independent component analysis-mixture model (VIM), an unsupervised machine-learning classifier to analyze frequency doubling technology (FDT) perimetry data.[31]
Combined Approaches
Oh et al used an artificial neural network (ANN) to predict OAG with an accuracy of 84.0%, sensitivity of 78.3%, and specificity of 85.9%.[32] Their ANN evaluated four non-ophthalmic factors (sex, age, menopause, and duration of hypertension) and five ophthalmic factors (IOP, spherical equivalent refractive errors, vertical cup-to-disc ratio, presence of superotemporal RNFL defect, and presence of inferotemporal RNFL defect). They suggest that visual fields, being cumbersome and impractical for screening, can be avoided with this approach.
Predicting the Prognosis
For visual field progression analysis, Goldbaum et al used Progression of Patterns (POP), a machine learning classifier algorithm.[33] Yousefi et al showed that machine learning detects VF progression consistently, without confirmation visits and even slow progression.[34] All these methods can ideally run on portable perimetry devices like the smartphone based virtual reality perimetry such as PeriScreener, VirtualEye, and C3FA.[35]
Wen, Lee and colleagues trained a deep learning system with 32,443 visual fields (24-2 VFs) taken between 1998 to 2018 and the resulting CascadeNet-5 model was able to predict future visual fields for up to 5.5 years based on a single input visual field.[36] Kazemian et al developed and validated Kalman Filters which could predict personalized trajectory of progression of Mean Deviation of Visual Fields at different target IOPs.[37] This would be a helpful guide for customized target IOP.
Future Prospects
The algorithms are always becoming better, and in the near future we can expect to have AI that has extremely good sensitivity and specificity that cannot be achieved by a human ophthalmologist. Combining the results of the various glaucoma evaluation tests might be the most promising to achieve a perfect diagnosis and prognosis, but the most convenient would be if a single test can be extrapolated using AI to predict the other. For example, if a single OCT can predict the visual field using AI for extrapolation, that would reduce the time required for a confirmatory diagnosis and thus allow early diagnosis and treatment.
Additional Resources
- Cybersight Consult from Orbis International incorporates the Pegasus AI system
- Artificial Intelligence in Retina
- Artificial Intelligence
- Artificial Intelligence in Glaucoma
- Article on Role of Artificial Intelligence in Ophthalmology
- Artificial Intelligence in Cataract Screening
- Article on Role of Artificial Intelligence in Cataract screening
References
- ↑ Jump up to: 1.0 1.1 1.2 Akkara JD, Kuriakose A. Role of artificial intelligence and machine learning in ophthalmology. Kerala J Ophthalmol 2019;31:150-60. doi: 10.4103/kjo.kjo_54_19.
- ↑ Akkara JD, Kuriakose A. Commentary: Rise of machine learning and artificial intelligence in ophthalmology. Indian Journal of Ophthalmology. 2019;67(7):1009. doi:10.4103/ijo.IJO_622_19
- ↑ Kapoor R, Whigham BT, Al-Aswad LA. The Role of Artificial Intelligence in the Diagnosis and Management of Glaucoma. Curr Ophthalmol Rep. 2019;7(2):136-142. doi:10.1007/s40135-019-00209-w
- ↑ Artificial Intelligence. Accessed May 29, 2020. https://www.lexico.com/definition/artificial_intelligence
- ↑ Machine Learning. Accessed May 29, 2020. https://www.lexico.com/definition/machine_learning
- ↑ Neural Network. Accessed May 29, 2020. https://www.lexico.com/en/definition/neural_network
- ↑ Deep Learning. Accessed May 29, 2020. https://en.wikipedia.org/wiki/Deep_learning#Definition
- ↑ Akkara JD, Kuriakose A. Role of artificial intelligence and machine learning in ophthalmology. Kerala Journal of Ophthalmology. 2019;31(2):150. doi:10.4103/kjo.kjo_54_19
- ↑ Zheng C, Johnson TV, Garg A, Boland MV. Artificial intelligence in glaucoma. Current Opinion in Ophthalmology. 2019;30(2):97. doi:10.1097/ICU.0000000000000552
- ↑ Martin KR, Mansouri K, Weinreb RN, et al. Use of Machine Learning on Contact Lens Sensor-Derived Parameters for the Diagnosis of Primary Open-angle Glaucoma. Am J Ophthalmol. 2018;194:46-53. doi:10.1016/j.ajo.2018.07.005
- ↑ Li Z, He Y, Keel S, Meng W, Chang RT, He M. Efficacy of a Deep Learning System for Detecting Glaucomatous Optic Neuropathy Based on Color Fundus Photographs. Ophthalmology. 2018;125(8):1199-1206. doi:10.1016/j.ophtha.2018.01.023
- ↑ Al-Aswad LA, Kapoor R, Chu CK, et al. Evaluation of a Deep Learning System for Identifying Glaucomatous Optic Neuropathy Based on Color Fundus Photographs. J Glaucoma. Published online June 21, 2019. doi:10.1097/IJG.0000000000001319
- ↑ Cerentini A, Welfer D, Cordeiro d’Ornellas M, Pereira Haygert CJ, Dotto GN. Automatic Identification of Glaucoma Using Deep Learning Methods. Stud Health Technol Inform. 2017;245:318-321.
- ↑ Haleem MS, Han L, Hemert J van, et al. A Novel Adaptive Deformable Model for Automated Optic Disc and Cup Segmentation to Aid Glaucoma Diagnosis. J Med Syst. 2017;42(1):20. doi:10.1007/s10916-017-0859-4
- ↑ Thompson AC, Jammal AA, Medeiros FA. A Deep Learning Algorithm to Quantify Neuroretinal Rim Loss From Optic Disc Photographs. Am J Ophthalmol. 2019;201:9-18. doi:10.1016/j.ajo.2019.01.011
- ↑ Retinal Image Analysis - Glaucoma. Accessed May 6, 2020. http://www.kalpah.com/RIAG_brochure.pdf
- ↑ Cifuentes-Canorea P, Ruiz-Medrano J, Gutierrez-Bonet R, et al. Analysis of inner and outer retinal layers using spectral domain optical coherence tomography automated segmentation software in ocular hypertensive and glaucoma patients. PLoS ONE. 2018;13(4):e0196112. doi:10.1371/journal.pone.0196112
- ↑ Omodaka K, An G, Tsuda S, et al. Classification of optic disc shape in glaucoma using machine learning based on quantified ocular parameters. PLoS ONE. 2017;12(12):e0190012. doi:10.1371/journal.pone.0190012
- ↑ Muhammad H, Fuchs TJ, De Cuir N, et al. Hybrid Deep Learning on Single Wide-field Optical Coherence tomography Scans Accurately Classifies Glaucoma Suspects. J Glaucoma. 2017;26(12):1086-1094. doi:10.1097/IJG.0000000000000765
- ↑ Asaoka R, Murata H, Hirasawa K, et al. Using Deep Learning and Transfer Learning to Accurately Diagnose Early-Onset Glaucoma From Macular Optical Coherence Tomography Images. Am J Ophthalmol. 2019;198:136-145. doi:10.1016/j.ajo.2018.10.007
- ↑ Christopher M, Belghith A, Weinreb RN, et al. Retinal Nerve Fiber Layer Features Identified by Unsupervised Machine Learning on Optical Coherence Tomography Scans Predict Glaucoma Progression. Invest Ophthalmol Vis Sci. 2018;59(7):2748-2756. doi:10.1167/iovs.17-23387
- ↑ Barella KA, Costa VP, Gonçalves Vidotti V, Silva FR, Dias M, Gomi ES. Glaucoma Diagnostic Accuracy of Machine Learning Classifiers Using Retinal Nerve Fiber Layer and Optic Nerve Data from SD-OCT. J Ophthalmol. 2013;2013:789129. doi:10.1155/2013/789129
- ↑ Bizios D, Heijl A, Hougaard JL, Bengtsson B. Machine learning classifiers for glaucoma diagnosis based on classification of retinal nerve fibre layer thickness parameters measured by Stratus OCT. Acta Ophthalmol. 2010;88(1):44-52. doi:10.1111/j.1755-3768.2009.01784.x
- ↑ Larrosa JM, Polo V, Ferreras A, García-Martín E, Calvo P, Pablo LE. Neural Network Analysis of Different Segmentation Strategies of Nerve Fiber Layer Assessment for Glaucoma Diagnosis. J Glaucoma. 2015;24(9):672-678. doi:10.1097/IJG.0000000000000071
- ↑ Fu H, Xu Y, Lin S, et al. Segmentation and Quantification for Angle-Closure Glaucoma Assessment in Anterior Segment OCT. IEEE Trans Med Imaging. 2017;36(9):1930-1938. doi:10.1109/TMI.2017.2703147
- ↑ Niwas SI, Lin W, Bai X, et al. Automated anterior segment OCT image analysis for Angle Closure Glaucoma mechanisms classification. Computer Methods and Programs in Biomedicine. 2016;130:65-75. doi:10.1016/j.cmpb.2016.03.018
- ↑ Asaoka R, Murata H, Iwase A, Araie M. Detecting Preperimetric Glaucoma with Standard Automated Perimetry Using a Deep Learning Classifier. Ophthalmology. 2016;123(9):1974-1980. doi:10.1016/j.ophtha.2016.05.029
- ↑ Li F, Wang Z, Qu G, et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med Imaging. 2018;18(1):35. doi:10.1186/s12880-018-0273-5
- ↑ Goldbaum MH, Sample PA, Zhang Z, et al. Using unsupervised learning with independent component analysis to identify patterns of glaucomatous visual field defects. Invest Ophthalmol Vis Sci. 2005;46(10):3676-3683. doi:10.1167/iovs.04-1167
- ↑ Andersson S, Heijl A, Bizios D, Bengtsson B. Comparison of clinicians and an artificial neural network regarding accuracy and certainty in performance of visual field assessment for the diagnosis of glaucoma. Acta Ophthalmol. 2013;91(5):413-417. doi:10.1111/j.1755-3768.2012.02435.x
- ↑ Bowd C, Weinreb RN, Balasubramanian M, et al. Glaucomatous patterns in Frequency Doubling Technology (FDT) perimetry data identified by unsupervised machine learning classifiers. PLoS ONE. 2014;9(1):e85941. doi:10.1371/journal.pone.0085941
- ↑ Oh E, Yoo TK, Hong S. Artificial Neural Network Approach for Differentiating Open-Angle Glaucoma From Glaucoma Suspect Without a Visual Field Test. Invest Ophthalmol Vis Sci. 2015;56(6):3957-3966. doi:10.1167/iovs.15-16805
- ↑ Goldbaum MH, Lee I, Jang G, et al. Progression of patterns (POP): a machine classifier algorithm to identify glaucoma progression in visual fields. Invest Ophthalmol Vis Sci. 2012;53(10):6557-6567. doi:10.1167/iovs.11-8363
- ↑ Yousefi S, Kiwaki T, Zheng Y, et al. Detection of Longitudinal Visual Field Progression in Glaucoma Using Machine Learning. American Journal of Ophthalmology. 2018;193:71-79. doi:10.1016/j.ajo.2018.06.007
- ↑ Akkara JD, Kuriakose A. Review of recent innovations in ophthalmology. Kerala Journal of Ophthalmology. 2018;30(1):54. doi:10.4103/kjo.kjo_24_18
- ↑ Wen JC, Lee CS, Keane PA, et al. Forecasting future Humphrey Visual Fields using deep learning. PLOS ONE. 2019;14(4):e0214875. doi:10.1371/journal.pone.0214875
- ↑ Kazemian P, Lavieri MS, Oyen MPV, Andrews C, Stein JD. Personalized Prediction of Glaucoma Progression Under Different Target Intraocular Pressure Levels Using Filtered Forecasting Methods. Ophthalmology. 2018;125(4):569-577. doi:10.1016/j.ophtha.2017.10.033