All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Diagnostic Intervention
Background
This article discusses the specific applications of various orbital ultrasound modalities in informing diagnosis in neuro-ophthalmology. For a more in-depth discussion pertaining to the mechanism of these ultrasound modalities, please see the related EyeWiki pages: Ophthalmic ultrasound and Ultrasound biomicroscopy.
Description/Overview
Orbital and ocular ultrasonography typically has two modes: A-scan and B-scan.
Standardized A-scan in ultrasound is a one dimensional “amplitude” modulation scan and can be used quantitatively for measurements (e.g., axial length, extraocular muscle enlargement) and qualitatively for ultrasonic properties (e.g., high, medium, or low internal reflectivity in posterior segment mass lesions).
B-scan ultrasound is a two dimensional, cross-sectional “brightness” scan and can be used in conjunction with A-scan to evaluate posterior segment (e.g., choroidal lesions), ocular (e.g., optic disc drusen) and orbital pathology (e.g., orbital masses, thyroid eye disease, optic nerve sheath fluid). B-scans are commonly used in the setting of visual loss with poor view of the fundus (e.g., vitreous hemorrhage with suspected retinal detachment or media opacity precluding ophthalmoscopy).
Indications
Vitreous
Subarachnoid bleeding associated with vitreous hemorrhage, or Terson syndrome (See related: Terson's Syndrome) may obscure the view of the posterior segment and ultrasound might be useful to visualize potential posterior segment pathology.
Posterior Segment pathology
Posterior pole and optic nerve colobomas and posterior staphylomas (e.g, high myopia) can be visualized on ultrasound. (See related: Coloboma).
Choroidal (e.g., choroidal metastasis, melanoma, or osteoma) and retinal lesions (e.g., astrocytic hamartoma (RAH) of tuberous sclerosis) can be characterized by ultrasound. The RAH is typically not progressive and may show high reflectivity, a significant acoustic shadow, and focal elevation. Ultrasound can detect calcifications in retinal lesions (e.g., retinoblastoma, osteoma) that might help in the differential diagnosis.[1]
Optic Nerve
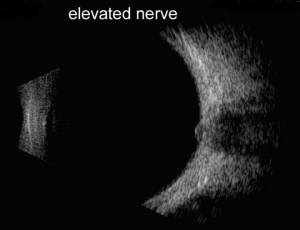
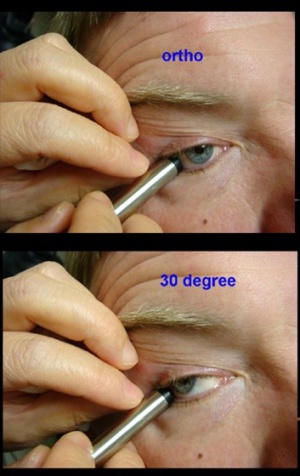
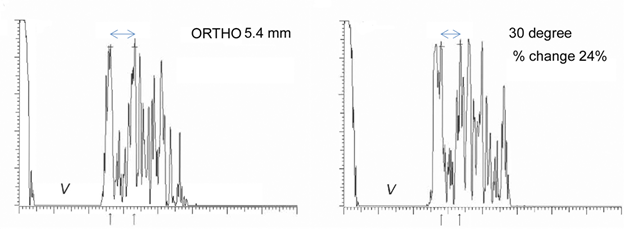
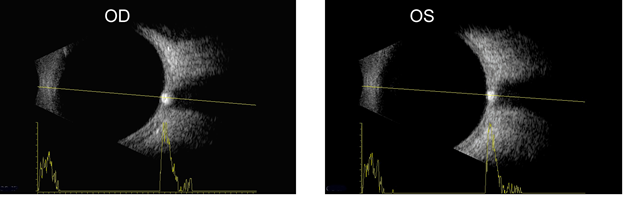
A and B-scan can be used to measure optic nerve thickness and reflectivity. The reported average thickness of optic nerve is 3.8mm[2], although in our clinic - 4.2 mm is the upper limit of normal. The optic nerve sheath diameter can be increased in elevated intracranial pressure. Repeating the ultrasound in primary position and 30 degrees away from primary can sometimes differentiate increased cerebrospinal fluid in the optic nerve sheath from increased optic nerve parenchymal diameter (30 degree test) with a change of 20% being significant.
In suspected trauma, B-scan can help to identify and characterize the type and location of intraocular or intraorbital foreign bodies. Orbital ultrasound can also identify intraorbital hemorrhage in trauma.
Papilledema vs. pseudopapilledema
Papilledema is optic nerve head swelling secondary to increased intracranial pressure and pseudopapilledema is elevation of the optic nerve head secondary to buried optic nerve head drusen or anomalous optic nerves. In a 2014 study of 87 patients by Carter et al., orbital ultrasonography had 90% sensitivity and 79% specificity for papilledema, compared to 90% and 67% respectively via lumbar puncture[2]. This is further supported by the 2018 study by Saenz et al reporting 91.3% sensitivity at 95% specificity for diagnosis of papilledema via A-scan ultrasound to measure optic nerve sheath diameter (ONSD) and perform the 30° test, compared to only 56.5% sensitivity using RNFL thickness measurement obtained via spectral-domain OCT[3]. A positive 30° sign is a reduction in ONSD by > 20% from primary gaze to 30° gaze, suggestive of the redistribution of fluid and increased intracranial pressure.
In a study comparing ultrasonography to magnetic resonance imaging (MRI) of the orbit, using a cutoff of ONSD >4.8mm in ultrasound had a sensitivity and specific of 77% and 82%, respectively, which was very comparable to MRI sensitivity and specificity of 77% and 83%, respectively using a cutoff of OSND > 6.0mm[4]. (See related: Papilledema and Pseudopapilledema). Wang et al. evaluated a cohort of 60 patients with suspected elevated intracranial pressure (ICP) found ONSD was strongly correlated with ICP, as was the subsequent decrease in ONSD and ICP on serial follow-up[5] .
Aside from papilledema diagnosis and follow-up, orbital ultrasonography can be used to support elevated ICP diagnosis in situations when:
- Papilledema may not manifest ophthalmoscopically but there is postpapilledema or other optic atrophy (e.g., persistent CSF in the optic nerve sheath and positive 30 degree test)
- Patients with a CSF shunt are suspected of shunt malfunction
- Patients who refuse or are unable to undergo a lumbar puncture for ICP measurement[6] .
Optic nerve head Drusen
Ultrasound can particularly be helpful in detection of calcified drusen in cases with pseudopapilledema. Ultrasound is less useful however in cases of non-calcified optic disc drusen.
Extraocular Muscle, Orbital Fat
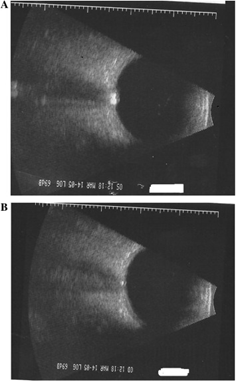
Autoimmune thyroid eye disease (ATED) is often characterized by enlarged muscle belly without thickening of the tendons. Early in the course of ATED the extraocular muscle thickness increases with low reflectivity, followed by fibrosis which corresponds to heterogeneous increase in reflectivity. Orbital adipose tissue may also show heterogeneous reflectivity. The thickened optic nerve in advanced ATED may be due to the compression of the nerve by the enlarged extraocular muscles[1].
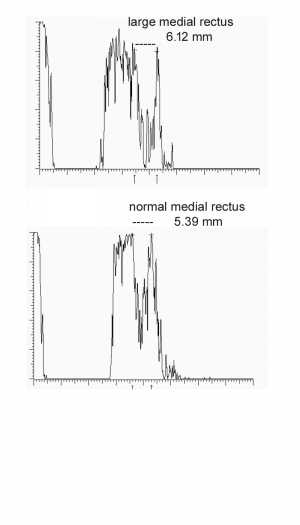
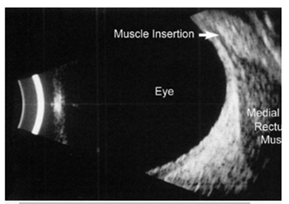
The upper end of normal extra-ocular width in our experience is 5.4 mm. When muscles are enlarged 5.7 mm and greater, confidence increases. Note that the muscle appears hollow, but this is due to tissue striations being parallel such that the sound waves are not distorted. The easiest muscle to measure is the medial rectus followed by the lateral rectus. Although the inferior rectus muscle is the first to be affected in thyroid eye disease, it is the most difficult to obtain reliably. The standardized A-scan measurements must be replicated several times as angle of incidence and eye movement cause significant variability. Lastly, mastering the Standardized A-scan has a steep learning curve.
In addition to axial length, A-scan can be used to assess orbital muscles for tissue edema and cellular infiltration based on changes in reflectivity. Internal muscle reflectivity is low during the active inflammatory phase of ATED but irregularly high during the end-stage fibrotic phase[7]. Visualization of the extraocular muscles via orbital B-scan enables evaluation of specific muscles for enlargement. Superior ophthalmic vein (SOV) congestion has been implicated in the inflammatory stage of ATED and color Doppler imaging (CDI) has been proposed for assessing for and temporally monitoring SOV congestion of ATED patients during the treatment timeline[8]. Additionally, CDI may be able to measure changes in central retinal and ophthalmic artery blood flow. Such blood flow velocities have been correlated with extraocular muscle enlargement in ATED [9].
Orbital Vessels
B-scan can be used to detect dilated orbital veins, which have low reflectivity, as do orbital hemangiomas[1]. Carotid-cavernous fistulas (CCF) are an abnormal connection between the arterial carotid system with the cavernous sinus, resulting in venous congestion and ocular manifestations such as exophthalmos, chemosis, conjunctival congestion and glaucoma. While the diagnostic gold standard for carotid-cavernous fistula (CCF) is digital-subtraction angiography (DSA), B-scan and color doppler ultrasound are noninvasive alternatives and has been used for screening and following patients with CCF. [10]. High-flow CCF has been diagnosed based on the ultrasound finding of a dilated (>2mm) superior ophthalmic vein (SOV) with reversed, arterialized, low-resistance blood flow. Visualization of a normal continuous SOV flow is indicative of a negative diagnosis of CCF.
Orbital Tumors
While optic nerve gliomas and optic sheath meningiomas can be detected on ultrasound, standard structural imaging (e.g., CT or MRI are superior for characterizing tumors in the orbit or optic nerve). Some orbital tumors, such as ocular melanomas (i.e. COMS), may still be visualized with ultrasound as an additional imaging modality to help to further characterize lesions.
Color Doppler Imaging (CDI)
CDI provides additional real-time data on vascular flow and directionality overlaid on B-scan. CDI enables evaluation of the major orbital arterial supply and venous drainage, namely the ophthalmic artery (OA) and superior ophthalmic vein, respectively[7]. CDI has been used to measure statistically significant decreased OA velocity in unilateral non-arteritic anterior ischemic optic neuropathy (NAION) compared to the non-involved contralateral eye[11]. CDI has been proposed for following patients with ischemia (e.g., giant cell arteritis (GCA) or serially monitor disease status via arterial blood flow) but requires further study[12].
Extra-orbital Ultrasound Studies in Neuro-Ophthalmology
Color doppler ultrasound is commonly used to evaluate the carotid and vertebral arteries (e.g., carotid or vertebral plaque morphology, stenosis or occlusion)which supply the entire visual pathway and visual cortex. Intracranial vessel flow can be assessed using ultrasound via transcranial doppler (TCD)[13]13. Blood flow velocities are recorded as grey scale spectrums. On the y-axis, blood flowing towards the transducer registers as positive.
TCD can be used in conjunction with CT imaging and duplex ultrasound to localize suspected occlusion or stenosis. Potential applications in neuro-ophthalmology are ischemic strokes with ophthalmic symptoms (e.g.,. amaurosis fugax) Possible other as yet unproven applications include AION due to occlusion of the short posterior ciliary artery, and PION due to occlusion of the posterior branches of the OA[14]. Pre-stenotic regions typically have normal spectrums, but can be more pulsatile and depressed with severe stenoses. Intra-stenotic regions have increased flow velocities that correlate with the severity of the stenosis. Post-stenotic regions have decreased velocities, increased turbulence with a retrograde component[14].
In summary, clinicians should be aware of the two main types of orbital/ocular ultrasound (A and B scan) and should recognize the clinical applications of this imaging modality for evaluating the posterior segment including the optic nerve (e.g., calcified disc drusen, 30 degree test for papilledema) as well as the orbit (e.g., orbital mass lesions, ATED, CCF).
References
- ↑ 1.0 1.1 1.2 Csákány,B. & Németh, J. The Role of the Ophthalmologic Ultrasound in Neuro-ophthalmology. in Neuro-Ophthalmology (eds. Somlai, J. &Kovács, T.) 253–256 (Springer International Publishing, 2016).
- ↑ 2.0 2.1 Carter,S. B. et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye 28, 1425–1430 (2014).
- ↑ Saenz,R., Cheng, H., Prager, T. C., Frishman, L. J. & Tang, R. A. Use of A-scan Ultrasound and Optical Coherence Tomography to Differentiate Papilledema From Pseudopapilledema. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 94,1081–1089 (2017).
- ↑ Patterson, D. F. et al.Comparison of Ocular Ultrasonography and Magnetic Resonance Imaging forDetection of Increased Intracranial Pressure. Front. Neurol. 9, (2018).
- ↑ Wang,L. et al. Ultrasonography Assessments of Optic Nerve Sheath Diameter as a Noninvasive and Dynamic Method of Detecting Changes in Intracranial Pressure. JAMA Ophthalmol. 136, 250–256 (2018).
- ↑ Lee,A. G. Correlation Between Noninvasive Ultrasonography and Dynamically Monitored Intracranial PressureCorrelation Between Noninvasive Ultrasonography andDynamically Monitored Intracranial PressureResearch. JAMA Ophthalmol. 136,256–256 (2018).
- ↑ 7.0 7.1 Gonçalves,A. C. P., Gebrim, E. M. M. S. & Monteiro, M. L. R. Imaging studies for diagnosing Graves’ orbitopathy and dysthyroid optic neuropathy. Clin. SaoPaulo Braz. 67, 1327–1334 (2012).
- ↑ Monteiro,M. L. R., Moritz, R. B. S., Angotti Neto, H. & Benabou, J. E. Color Doppler imaging of the superior ophthalmic vein in patients with Graves’ orbitopathy before and after treatment of congestive disease. Clin. Sao Paulo Braz. 66,1329–1334 (2011).
- ↑ Alp,M. N., Ozgen, A., Can, I., Cakar, P. & Gunalp, I. Colour Doppler imaging of the orbital vasculature in Graves’ disease with computed tomographic correlation. Br. J. Ophthalmol. 84, 1027–1030 (2000).
- ↑ Venturini,M. et al. Orbital color Doppler ultrasound as noninvasive tool in the diagnosis of anterior-draining carotid-cavernous fistula. Radiol. Med.(Torino) 121, 301–307 (2016).
- ↑ Sanjari,M. S., Falavarjani, K. G., Mehrabani, M., Ghiasian, L. & Zamani, B.Retrobulbar haemodynamics and carotid wall thickness in patients with non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol. 93,638–640 (2009).
- ↑ Ghanchi,. D. et al. Colour doppler imaging in giant cell (temporal) arteritis:Serial examination and comparison with non-arteritic anterior ischaemic optic neuropathy. Eye 10, 459–464 (1996).
- ↑ Pánczél, G. Transcranial Doppler Examination. in Neuro-Ophthalmology (eds. Somlai, J. & Kovács, T.)231–245 (Springer International Publishing, 2016).
- ↑ 14.0 14.1 Barsi,P. Duplex Ultrasound Examination of the Carotid and Vertebral Arteries. in Neuro-Ophthalmology(eds. Somlai, J. & Kovács, T.) 223–230 (Springer International Publishing,2016).