Suprachoroidal Medication Administration
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction/Injection Site
Suprachoroidal injections allow for precise, targeted delivery to the retina, RPE, and choroid [1][2], with animal studies showing a 12-fold higher drug delivery compared to intravitreal injections [3].
The suprachoroidal injection site is positioned approximately 4-4.5mm from the limbus in the superior temporal quadrant. This allows entrance into the suprachoroidal space, athin space between the sclera and choroid [4]. Uveal-scleral fluid flow within the compartmentalized space allows for drugs to reach target tissue with minimal unnecessary contact [1].
Suprachoroidal injections utilize a hollow microneedle with a depth set that solely punctures the sclera and conjunctiva to avoid inadvertent damage to the retina or choroid [5]. This microneedle allows for minimally invasive access to the suprachoroidal space and allows for novel, targeted therapy to the choroid and retina with minimal effect on the anterior segment [6][7].
Disease Treated by Suprachoroidal Injections
Macular edema associated with non-infectious uveitis is the only FDA approved indication for suprachoroidal drug injection.
Drugs Using Suprachoroidal Injections
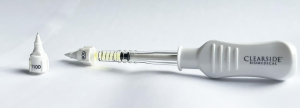
Triamcinolone acetonide (Xipere 40 mg/mL; Bausch & Lomb) is the first and only FDA approved drug to be injected in the suprachoroidal space [8].
The PEACHTREE and MAGNOLIA clinical trials have been pivotal in establishing the efficacy and safety of suprachoroidal injections for the treatment of uveitic macular edema.
The PEACHTREE trial, a randomized, double-masked, sham-controlled study, demonstrated that patients receiving suprachoroidal injections of triamcinolone acetonide (CLS-TA), at day 0 and week 12, showed significant improvement in macular edema and visual acuity compared to the sham group. Forty seven percent of the patients treated with suprachoroidal injections achieved at least a 15-letter improvement in best corrected visual acuity (BCVA) from baseline at week 24 [9].
The MAGNOLIA study extended the findings of the PEACHTREE trial by examining the long-term efficacy and safety of CLS-TA in treating macular edema associated with non-infectious uveitis. The results showed that median time to use of rescue medication relative to the last dose of study drug was longer in the CLS-TA arm compared with the control arm (257.0 days vs 55.5 days, respectively) [10].
Clinical trials are currently investigating additional drug therapies, including aflibercept, RGX-314 gene therapy, and belzupacap sarotalocan, effectiveness in the suprachoroidal space.
- Aflibercept: Aflibercept is an anti-vascular endothelial growth factor (VEGF) agent that has been shown to be effective in treating macular edema, especially associated with diabetic retinopathy. Barakat et al. conducted a prospective study, TYBEE, that examined the potential benefits of administering suprachoroidal triamcinolone acetonide with intravitreal aflibercept compared to aflibercept monotherapy [11]. TYBEE concluded that the combination therapy showed significant anatomical improvements in central subfield retinal thickness but only a slight, non-significant improvement in visual benefit [11]. Additional clinical trials are required to examine the benefits of aflibercept versus triamcinolone acetonide versus combination therapy.
- RGX-314 Gene Therapy: RGX-314 is a gene encoding for a monoclonal antibody fragment that binds and neutralizes VEGF and is being explored to reduce disease burden of life-long intraocular injection requirements in patients with wet age-related macular degeneration (AMD). It can be administered in a subretinal, or suprachoroidal manner [10]. A clinical trial that is evaluating its clinical utility is the AAVIATE trial, which is still in the recruitment phase.
- Belzupacap Sarotalocan/AU-011: AU-011 or belzupacap sarotalocan, has emerged as a potential alternative therapy for small choroidal melanoma. AU-011 is an investigational therapy consisting of viral-like drug conjugate that binds to heparan sulfate proteoglycans (HSPG) on the surface of tumor cells. It can be administered intraretinally or via suprachoroidal injection with phthalocyanine photosensitizer and is activated by a 689 nm wavelength ophthalmic laser [12]. AU-011 requires further research into its efficacy.
Information about suprachoroidal devices can be found here.
Other Techniques Used for Suprachoroidal Injections
Currently only the 900 or 1,100 um Xipere microneedles are FDA approved for suprachoroidal administration. Three other techniques for suprachoroidal injections include: microcatheters, needles, and microneedles [1].
- Catheter-based technology involves inserting a 250 A microcatheter into the SCS, which has precise targeting and visualization due to flashing diode but is invasive and dependent on skill of administrator
- Free hand use of standard hypodermic needle attached to a Hamilton syringe or insulin syringe through sclera behind limbus, which is more accessible and less invasive but lacks visualization and requires high level skill
- Hollow microneedles used for transdermal drug delivery with varying microneedle length dependent on scleral thickness, which is both precise and accessible as it can be done in an office setting due to limited penetration.
A case report showed efficacy of using custom made 30-gauge needle with 1000-micron penetration of the sclera at the pars plana with gentle pressure on the sclera for single injection of triamcinolone acetonide for macular edema due to retinal vein occlusion [13].
Risks and Informed Consent of Suprachoroidal Injections
The risks, benefits, indications, and alternative therapies should be thoroughly discussed with patients. Informed consent should be obtained prior to any injections. Suprachoroidal injections have shown to similar safety profiles than intravitreal injections [14].
Risks include:
- Patient discomfort.
- Bleeding: subconjunctival or suprachoroidal hemorrhage.
- Vitreous detachment: noted in 5% in Phase III clinical trial [9].
- Retinal detachment [9].
- Post-injection IOP elevation: noted in 12-14% of patients in the PEACHTREE trial. However, IOP was noted to return back to baseline within 1 hour of injection [9].
- Cataracts: noted to develop in 6-7% of treated and sham-treated participants respectively [9].
- Endophthalmitis: a rare adverse effect with an incidence rate of 1 in 2000-5000 for intravitreal injections [15] and an unknown incidence rate in suprachoroidal injections.
- Worsening of active infectious uveitis: an infection should be thoroughly ruled out prior to initiating steroid injections. Case series have shown that treating misdiagnosed infectious uveitis with intravitreal steroid injections can lead to poor visual acuity outcomes [16].
- Vision loss: due to any of the above complications.
- Need for surgery: to address any of the above ocular complications.
- Eye loss: due to severe infection.
Benefits:
Prevention of worsening of vision and potential improvement in vision.
Indications:
Macular edema associated with non-infectious uveitis.
Alternative Therapies
No treatment with observation, intravitreal injections, topical corticosteroids, systemic corticosteroids, corticosteroid implants, biological immunomodulatory therapies, pars plana vitrectomy.
Preparation for Injection
Positioning:
Supine position with secure head support. A sterile lid speculum may be useful to keep lids open during injection as injection time is significantly longer than intravitreal injections and may require both hands. More about intravitreal injection technique can be found here.
Environment:
Conducted in an outpatient setting. Patient should be in a quiet room with the door closed. All instruments should be prepared prior to injection. A timeout should be done to confirm the correct patient, injection, eye, and any allergies.
Anesthetic:
Subconjunctival or topical anesthetics, such as 2% lidocaine, can be used prior to injection to decrease discomfort. While subconjunctival anesthesia may reduce pain better than topical anesthetics, it may increase conjunctival thickness making it more difficult to approximate the microinjector against the globe of the eye [17].
Antiseptic:
Topical antiseptic, such as 5% betadine, should be used prior to sterile marking and injection.
Microneedle preparation:
900 um microneedles are more commonly utilized and preferred for initial suprachoroidal injections. A longer 1,100 um microneedle may be needed in some patients if resistance is felt indicating inadequate penetration of the suprachoroidal space [1].
Injection Site
- Approximately 4-4.5mm from the limbus in the superior temporal quadrant.
- A space between the sclera and the choroid, extends anteriorly to the scleral spur near ciliary body and posteriorly near optic nerve
- Needle inserted through sclera to reach space
- SCS has an approximated thickness of 35 μm
- Relatively thin space due to IOP that can tolerate expansion with fluid without lasting impact
- Uveal-scleral fluid flow allows for drug microparticles to travel to posterior region of space
- Compartmentalization in space allows focused exposure to target tissues and minimizing extra contact with anterior segment
Injection Technique
- Confirm informed consent.
- Timeout to confirm correct patient and eye.
- Apply topical or subconjunctival anesthetic.
- Apply a topical, local anesthetic to prevent ocular infection prior to marking and injection.
- Mark and confirm selected injection site.
- Align the microinjector perpendicular to the injection site to reach the suprachoroidal space. Perpendicularity should be confirmed by many viewing angles.
- Insert the microneedle, being sure to create a small dimple on the ocular surface to allow the tissue to displace gently while the needle enters the suprachoroidal space (18).
- Inject slowly and deliberately over 5-10 seconds to decrease patient discomfort. Patients may feel the rapid expansion of the suprachoroidal space if the drug is injected too quickly. This slow and steady technique, while maintaining a perpendicular angle, may be best achieved using two hands for support.
- After finishing drug injection, hold the microinjector in place for an additional 3-5 seconds. Light pressure should be applied with a cotton swab after microinjector removal for about 5 seconds.
- Rinse and clean iodine off the patient’s eye.
- Check IOP after injection. Some providers may monitor for IOP reduction to a reasonable level before a patient leaves.
*Same day bilateral suprachoroidal injections may be done; however, both eyes should be treated as separate procedures with different medication vials and injectors.
Post Injection Care
Intraocular pressure should be checked after injection. Patients should be counseled on common post-injection side effects including subconjunctival hemorrhage and eye soreness. Patients should also be warned about red flag symptoms for more serious adverse effects including vision changes, eye redness, sensitivity, or pain that should prompt urgent follow-up with an ophthalmologist.
Treatment Care & Followup
Patients should regularly follow with their ophthalmologist to assess for improvement or resolution of intraocular edema. Treatment courses may vary but repeat treatments for suprachoroidal triamcinolone acetate (Xipere) should not be conducted until 12 weeks after the prior dose [8].
Additional Sources
- Video showing suprachoroidal injection technique: https://eyetube.net/videos/suprachoroidal-space-scs-injection-technique
- Video showing suprachoroidal injection technique with a custom needle: https://www.aao.org/education/clinical-video/simple-method-suprachoroidal-injection-with-custom
- Review article of suprachoroidal injections: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10535603/
- Xipere microinjector instructions: https://www.xipere.com/hcp/scs-microinjector/
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Wu KY, Fujioka JK, Gholamian T, Zaharia M, Tran SD. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals (Basel). 2023;16(9).
- ↑ Thomas J, Kim L, Albini T, Yeh S. Triamcinolone acetonide injectable suspension for suprachoroidal use in the treatment of macular edema associated with uveitis. Expert Rev Ophthalmol. 2022;17(3):165-73.
- ↑ Muya L, Kansara V, Cavet ME, Ciulla T. Suprachoroidal Injection of Triamcinolone Acetonide Suspension: Ocular Pharmacokinetics and Distribution in Rabbits Demonstrates High and Durable Levels in the Chorioretina. J Ocul Pharmacol Ther. 2022;38(6):459-67.
- ↑ Wan CR, Kapik B, Wykoff CC, Henry CR, Barakat MR, Shah M, et al. Clinical Characterization of Suprachoroidal Injection Procedure Utilizing a Microinjector across Three Retinal Disorders. Transl Vis Sci Technol. 2020;9(11):27.
- ↑ Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Advanced Drug Delivery Reviews. 2018;126:58-66.
- ↑ Patel TH, Marcus L, Horiba MN, Donoghue M, Chatterjee S, Mishra-Kalyani PS, et al. FDA Approval Summary: Pemigatinib for Previously Treated, Unresectable Locally Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusion or Other Rearrangement. Clin Cancer Res. 2023;29(5):838-42.
- ↑ Jung JH, Chae JJ, Prausnitz MR. Targeting drug delivery within the suprachoroidal space. Drug Discov Today. 2019;24(8):1654-9.
- ↑ Jump up to: 8.0 8.1 XIPERE (triamcinolone acetonide injectable suspension) [package insert]. Bausch + Lomb; 2021.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 Yeh S, Khurana RN, Shah M, Henry CR, Wang RC, Kissner JM, et al. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology. 2020;127(7):948-55.
- ↑ Jump up to: 10.0 10.1 Khurana RN, Merrill P, Yeh S, Suhler E, Barakat MR, Uchiyama E, et al. Extension study of the safety and efficacy of CLS-TA for treatment of macular oedema associated with non-infectious uveitis (MAGNOLIA). Br J Ophthalmol. 2022;106(8):1139-44.
- ↑ Jump up to: 11.0 11.1 Barakat MR, Wykoff CC, Gonzalez V, Hu A, Marcus D, Zavaleta E, et al. Suprachoroidal CLS-TA plus Intravitreal Aflibercept for Diabetic Macular Edema: A Randomized, Double-Masked, Parallel-Design, Controlled Study. Ophthalmol Retina. 2021;5(1):60-70.
- ↑ Wu F, Lane AM, Trofimov A, Shih HA, Gragoudas ES, Kim IK. Outcomes after Proton Beam Irradiation in Patients with Choroidal Melanoma Eligible for Investigational AU-011 Treatment. Ocul Oncol Pathol. 2023;9(5-6):152-7.
- ↑ Marashi A. Treating macular edema secondary to retinal vein occlusion with suprachoroidal injection of triamcinolone acetonide using custom made needle. Advances in Ophthalmology & Visual System. 2018;8(5).
- ↑ Abdelshafy Tabl A, Tawfik Soliman T, Anany Elsayed M, Abdelshafy Tabl M. A Randomized Trial Comparing Suprachoroidal and Intravitreal Injection of Triamcinolone Acetonide in Refractory Diabetic Macular Edema due to Epiretinal Membrane. J Ophthalmol. 2022;2022:7947710.
- ↑ Gregori NZ, Flynn HW, Jr., Schwartz SG, Rosenfeld PJ, Vaziri K, Moshfeghi AA, et al. Current Infectious Endophthalmitis Rates After Intravitreal Injections of Anti-Vascular Endothelial Growth Factor Agents and Outcomes of Treatment. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):643-8.
- ↑ Kim JMC, D.; Martin, D. F.; Srivastava, S. . Clinical Outcomes Intravitreal and Peri-Ocular Steroid Injections in Misdiagnosed Infectious Uveitis. nvestigative Ophthalmology & Visual Science. 2010;51.
- ↑ Villafuerte-Trisolini CY, G. A beginner’s guide to suprachoroidal injections. Retina Specialist. 2023.